Scientific Journal of Genetics and Gene Therapy
Detection of new mutations in 3 cases de novo tuberous sclerosis
Carlos Andrés Quintero Vásquez1, Isabel Fernández González2, María Luisa Quevedo Camera1, Angélica María García Ordoñez1 and Luis Gustavo Celis Regalado3*
2Medical genetics unit, Metropolitan Polyclinic Caracas, Venezuela
3MSC, Faculty of Medicine, University of La Sabana, Chia, Colombia
Cite this as
Quintero Vásquez CA, González IF, Quevedo Camera ML, García Ordoñez AM, Celis Regalado LG (2019) Detection of new mutations in 3 cases de novo tuberous sclerosis. Scientific J Genet Gene Ther 5(1): 001-006. DOI: 10.17352/sjggt.000017Introduction: The tuberous sclerosis complex is a multisystemic disease of autosomal dominant etiology, not pretty common in Latin America. It is caused by the mutation of the TSC1 and TSC2 genes which is characterized by uncontrolled production of Hamartomas in diverse organs and systems. However, some patients could present asymptomatic characteristics whereas other could experience fatal symptoms.
Objective: The scope of this work aims the process of reviewing inside the framework of 3 cases of tuberous sclerosis, reported at the Unidad genetica policlinica metroplitana of Caracas, Venezuela (Metropolitan polyclinic genetics unit in Caracas Venezuela).
Methodology: Diverse approaches were performed: A genetic medical report, a family genealogy, physical examination, complementary paraclinical analyses and DNA sequencing studies by PCR.
Results: Three cases de novo mutations of tuberous sclerosis reported, that have not published national neither Latin American. These cases were identified at the Metropolitan polyclinic genetics unit in Caracas Venezuela. One of the cases was a prenatal diagnosis in the 30th week of pregnancy by ultrasound control associated with the presence of cardiac rhabdomyomas.
Other two cases had postnatal diagnosis, related to different manifestations of tuberous sclerosis confirmed by DNA sequencing identifying different mutations of the TSC1 and TSC2 genes (TSC2: variant 1, 4 bp deletion, with nucleotide in position 4544-4547, codon 1515-1516; TSC1, c.509-1G-> A, splice IVS 6; TSC1 gene exon 15, c.1782_1786delGGGCT).
Introduction
Tuberous sclerosis is an inherited autosomal dominant disease, with high penetrance and very variable phenotypic expression, as a result of defects in hamartin production (TSC1) in the locus of chromosome 9 and tuberin (TSC2) in the locus of chromosome 16, this one have an inhibitory effect on growth cells [1], multisystemic involvement and high phenotypic variability. This condition has a global estimated incidence of 1/10,000 to 1/50,000 and two out of three cases have a sporadic presentation, which can appear at early ages or adulthood [2,3]. Tuberous sclerosis established the prototype of malformation related to differentiation and cell growth. Also, it presents with variable phenotypes that have systemic implications associated with dysplasia in diverse tissues, neoplasms, and other morphogenic alterations [1,2,4-6].
In 1835 the first description of skin lesions was published by Pierre Fransois Olive, who reported a case of multiple facial angiofibromas and erythematous papules. These were called sebaceous adenomas [2,5]. Subsequently, in 1862, Friedrich Daniel Von Recklinghausen, presented at the Berlin Obstetrics Society a newborn who death few hours after the delivery. He found cardiac lesions and brain sclerotic injuries without any association of these two pathologies [1,2]. Afterwards, in 1880, the first descriptions of cerebral tuberous sclerosis were reported by Desire Maloire Bourneville, who then determined the descriptions of cerebral pathology and neurological signs of tuberous sclerosis. At the beginnings of the 20th century, additional clinical and radiological information about this disease was obtained; also the association between the brain, kidney, cardiac and dermatological lesions were recognized in 1905, forming the Vogt triad (epilepsy, mental retardation and sebaceous adenoma) [1,2,4-6].
Methodology
A parent interviews were conducted to redact a prenatal, natal and postnatal report. Furthermore a genealogy, an exhaustive physical exam, evaluations by neuropediatrics and ophthalmology with complementary paraclinical analyses such as nuclear magnetic resonance imaging (MRI), fetal echocardiogram and DNA sequencing.
Results
Case 1
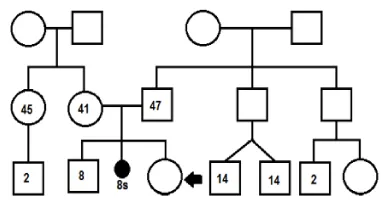
Consisted of a patient who had a prenatal diagnosis at gestational period of 30th weeks, ultrasound doppler findings showed two radiopaque nodules suggestive of rhabdomyomas at the level of the left ventricle. This was product of IIIG, IA, the mother was 41 years old and father who was 47 years old, healthy, non-consanguineous couple. Without history of inherited genetic disease up to three generations (Figure 1), genetic amniocentesis 46 XX.
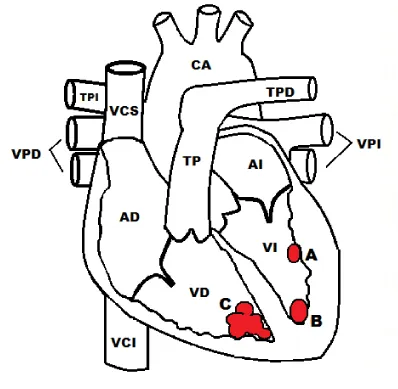
After a vaginal delivery, the newborn cried and breathed spontaneously, without cardiovascular complications during the natal and postnatal period. Afterward, a fetal echocardiogram was performed with oval images adhered to the left ventricular apical wall (Figure 2A) and free (Figure 2B), rounded and multiple, refractory ovoid images with ultrasound features of rhabdomyomas in the Apical right ventricle (Figure 2C). Those were without hemodynamic impact.
In the physical exam 21 days after being born evidenced a normocephalic, linear erythematous lesion at interciliary region, presence of cataract in the right eye, no dermal lesions were found also a neurological examination with normal limits according to age.
As a result, the clinical impressions of hamartosis and tuberous sclerosis were under study. The Nuclear magnetic resonance imaging in the skull such a MRI, electroencephalogram (EEG), assessment by neuropediatrics, abdominal, cerebral echocardiography, and molecular biology studies for tuberous sclerosis were ordered.
Fifty days after birth, a right phacoemulsification was performed with a 21.5 diopter lens. The patient showed a convulsive episode after that event, a skull MRI was performed 75 days after born with images corresponding to multiple subependymal nodules, hyperintense lesions in the white substance of T1, EEG with an asymmetry of frequencies and amplitude expense of the left temporal region, without paroxysmal abnormalities. A decision to start treatment was done with valproic acid. Other analyses, such as cerebral and abdominal echocardiography were performed without additional findings.
A new physical examination was performed 90 days after born, which reveals the appearance of multiple hypochromic maxims distributed throughout the body, with right lateralization. Ophthalmology made a new assessment that showed a cyclic membrane of the right eye after the phacoemulsification meriting vitrectomy via pars plana.
DNA sequencing studies were done seeking for mutations related to tuberous sclerosis. A TSC1 were found without deletions or duplications detected and no abnormal DNA sequences were identified, TSC2 with a disease-like DNA variant associated with mutation, with the presence of variant 1, 4 bp deletion, with nucleotide at position 4544-4547 codon location in 1515-1516, which confirms the patient diagnosis.
Case 2
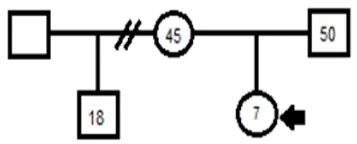
A seven years old and seven months male patient with routinely control during pregnancy, delivery without complications full-term. The mother was 38 years IIG and healthy. The father with a history of epidermal cyst and infertility. They were neither a consanguineous couple nor probable neither remote without a history of the inherited genetic disease known up to three generations (Figure 3).
The Newborn obtained by cesarean, breathed and cried spontaneously at birth: His weight 3385 grams, size 52cm with a neonatal period without complications. No alterations at psychomotor development. At the time of the assessment, the patient was starting second grade of primary school, with adequate school performance according to his age.
Since birth, the patient showed hypochromic lesions with a progressive increase of their size and number. Due to this he has been evaluated by a dermatologist, who consider that the patient presents a tuberous sclerosis illness. The physical exam evidenced normocephalic phenotype with multiple hypochromic macules disseminated throughout the body, with neurological examination without alterations, according to his age.
The PCR amplification, a DNA sequencing study was performed, with evidence of IVS 6 alteration in the TSC1 gene, c.509-1G-> A, located at the IVS 6 junction site without additional mutations. Findings are compatible with the diagnosis of tuberous sclerosis complex.
Case 3
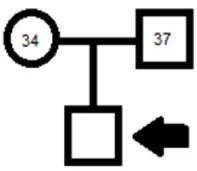
A 7 months old Male patient, who attended to the genetic consultation in order to be evaluated. It was a routinely control during the pregnancy with a full-term as a result of the first gestation (G1A0). Pregnancy was considered under high risk due to maternal hypothyroidism, on treatment over a 5 years prior to pregnancy. Healthy father. They were neither consanguineous couple nor likely remote, with no history of hereditary genetic disease known up to three generations (Figure 4), fetal echocardiogram at 33 weeks of pregnancy reported a structurally normal heart with evidence of supraventricular extra systoles.
Newborn obtained by cesarean by maternal decision. A patient who cried and breathed spontaneously at birth. Weight 3655 grams, size 52cm, without cardiovascular alterations during the neonatal period.
After 16 days of being born, a control echocardiogram was performed with evidence of multiple intracardiac rhabdomyomas without hemodynamic repercussions. Therefore, tuberous sclerosis was suspected and control assess performed by neuropediatrics who did not evidence neurological deficit. However, a brain NMR showed the presence of small nodules about the wall of the left lateral ventricle. In the other hand, ophthalmology assessment was performed without any compromise.
The patient’s phenotype at the evaluation time had 46.5cm of head circumference, dolichocephaly, wide forehead, multiple hypochromic macules distributed throughout the body, hyperchromic macula in the left thigh, with neurological examination according to age. Consequently the diagnosis of Hamartosis was considered under study: Tuberous sclerosis type I. Subsequently, a study of DNA sequencing of the TSC1 gene and deletion was carried out which showed in heterozygous patient alteration in exon 15 of the TSC1 gene c.1782_1786delGGGCT, without exon deletion, and the diagnosis of tuberous sclerosis is confirmed.
Discussion
The Tuberous Sclerosis Complex is a disorder that results from mutations in the TSC1 genes (chromosome 9q34), consisting by 23 exons which encode proteins of 130 kDa and TSC2 (chromosome 16p13.3), forming 41 exons, covering 45Kb of DNA genomic and encodes 5.5Kb of mRNA [3,5,7-9]. The mutation in the TSC1 gene inactivates the hamartin protein, while the TSC2 gene disorder alters the function of the tuberin protein, tumor suppressors, which is widely distributed in the brain, kidney, heart among others [3,5-14].
Both proteins form a heterodimeric TSC complex which integrates the ascending signal inputs such as growth factors, cellular nutrients, energy levels, stress, oxygen, and cytokines, which are altered [1,8].
As a consequence of this modification of the TSC1 and TSC2 genes prevents the suppression of Rheb (Ras homologue enriched in the brain) that in its active state (together with guanosine triphosphate) stimulates mTORC1 (mammalian target of the rapamycin 1 complex) [12] and its activation conducts multi-faceted cascade events such as MARN translation control, glycolysis, lipid synthesis, the pentose phosphate pathway and de novo pyrimidine synthesis acting as an important growth factor, that triggers the uncontrolled production of hamartomas throughout the body [8].
The hamartomas growth in tuberous sclerosis requires the inactivation of both alleles of TSC1 or TSC2 [7,8]. This loss of heterozygosity is assumed to be caused by an independent somatic mutation, according to the Knudson hypothesis [7,8,11-14].
A study of 325 individuals diagnosed with tuberous sclerosis identified that 50% of the patients presented a mutation in the TSC2 gene, 17% of the mutations were found in the TSC1 gene, 4% in unclassified variants and 29% without the identified mutation (INM) [15].
Patients affected by this pathology have a wide range of secondary clinical manifestations into the range of organs and systems affected by uncontrolled formation of hamartomas, such as skin, retina, kidneys, heart, lung and central nervous system [1-5,13]. The last one generates the highest morbidity and mortality with infantile spasms, intractable epilepsy, cognitive disability and autism being the most frequent, considering the fact that the characteristic features of the disease are not present from birth, and can manifest at different stages of life [3,5-7,10,16].
Despite the above mentioned, some patients have been found with the presence of lesions in a single organ or system, which sometimes make difficult the diagnosis and it should be done using molecular techniques [17].
The classic Vogt triad consists of the presence of angiofibromas, mental retardation and epilepsy. However, it is found only in 29% of the total patients [18,19]. Our patients had found lesions in the skin, brain, and heart, which, according to the literature the first two are the most frequent [1-7,10,16] and only in one of our cases, brain lesions are manifested and required pharmacological treatment.
Lesions that affect the cardiovascular system symptomatology may vary depending on the location and tumour size, being asymptomatic the majority of them [1,3,5,20,21]. These are secondary to the production of rhabdomyomas dependent on myocardial muscle fibers, and the diagnosis is made by echocardiography [19,22]. These lesions require invasive treatment for people with severe symptoms in the neonatal period when the tumor size is extreme [22,23].
The main cause of adults mortality with tuberous sclerosis are renal complications in 70 to 90% of adult patients which have multiple bilateral angiomyolipomas. This tumor, consists of abnormalities of the vessels, smooth muscle, and adipose tissue. It has an average growth of 3cm to 4cm every two years [4].
The clinical diagnostic criteria established in the consensus conference of the tuberous sclerosis complex [24], are divided into major and minor criteria (Table 1), the diagnosis is made it with two major criteria or one major and two minor criteria. The suspicion of a probable case with major and minor criteria and a possible case with major criteria and two or more minor criteria [10,18,23,25].
The final diagnosis is made it with the identification of genetic mutations in TSC1 and/or TSC2 [1,2,5,22-24], are different techniques for identifying them. The sequencing of the TSC2 gene allows mutations to be detected in 60%-70% of sporadic cases and in 50% of relatives, while mutations are detected in the TSC1 gene in 15% and 30% of sporadic and familial cases. Other techniques such as MLP (Multi-Layer Protocol), FISH (Fluorescence in Situ Hybridization), or Southern blot are used when the analysis by sequencing is negative, which allows identifying mutations in 0.5% to 5% of the cases (two).
The goals of treatment for people with tuberous sclerosis are the same as for all people with a chronic multisystemic disease, to provide the best possible quality of life with the least number of complications of the underlying disease, the least number of adverse effects of the treatment and the least amount of medications [26] and that is why the treatment is started in all patients with a family history of 3 generations and looking for relatives who are in a possible risk [25].
Subsequently, the management should be focused according to the affected organs that each patient presents, however, cerebral, thoracoabdominal and EEG MRI must be performed. Moreover dermatologic, dentist and ophthalmology assess which should be performed periodically, to identify prematurely lesions and give the appropriate medical and surgical management [2,5,25].
If the patient has seizures, treatment should be initiated faster and aggressively, avoiding complications, vigabatrin has being the medication of choice, but it should be used with caution since it has demonstrated retinal toxicity, resulting in visual field defects or loss of visual acuity potentially irreversible in 30% to 40% of patients who use it. Therefore, systemic corticosteroids can be used as a second line of management, besides, lamotrigine or felbamate that may also be useful depending on the type of seizure and the treatment response [26].
However, the rate of epilepsy refractory to medical treatment in patients with tuberous sclerosis is 50%-80% [26,27]. For these patients, a ketogenic diet or carbohydrate restriction alone and vagus nerve stimulation could be an option [26-28].
Resection surgery for epileptogenic lesion (s) is another treatment option that should be considered for refractory epilepsy, due to it offers significant benefits minimizing or ending the seizures [26].
Dermatological lesions are not associated with a significant risk of malignant transformation, but treatment can be considered if it is considered necessary or desirable by patients for cosmetic reasons [26].
Facial angiofibromas can be treated with laser therapy, dermal abrasion, or surgical excision. Fibrous plaques, nail fibroids and Shagreen patches can be treated with laser or surgical therapy [26].
On the other hand, patients who have a systemic and progressive disease could have some benefits with other therapies such mTOR inhibitors; however the decision of taken these therapeutic options must be selected in some individual cases taken in consideration benefits and risks [11,29].
The prognosis of patients is variable, and it depends on the clinical manifestations, progression, and hamartomas location. Patients who have not developed cerebral anatomical lesions and any neurological symptom by five years have a favorable intellectual prognostic [3] and cases with the presence of cardiac rhabdomyomas without significant neurological lesions [22,30,31]. However, a worse neurological prognosis of the mutations in TSC2 has been documented, with a higher percentage of drug-resistant epilepsy, more severe cognitive retardation, a higher frequency of behavioral disturbances and a greater load of NMR lesions, as well as other neuropsychiatric disorders [1,31] and in cases with association with Systemic Lupus Erythematosus [32,33].
Finally, it is concluded that the tuberous sclerosis complex is a rare entity in Latin America, multisystemic, of autosomal dominant etiology, whose initial diagnosis is clinical and the relevant studies must be carried out for diagnosis confirmation, and thus define the management guidelines therapeutic and preventive through a multidisciplinary team and be able to conclude genetic counseling and perform the calculation of recurrence risk in affected individuals.
- Monterio T, Garrido C, Pina S, Chorão R, Carrilho I, et al. (2014) Esclerosis tuberosa: caracterización clínica e intento de correlación fenotipo/genotipo. An Pediatr 81: 289-296. Link: http://bit.ly/2kIDjF1
- Medina C, Carreño O, Vélez A, Lizcano LA (2012) Complejo esclerosis tuberosa. Acta Neurol colomb 28: 11 - 23. Link: http://bit.ly/2kb8Q20
- Torres V, Contreras GA, Serrano N, Páez MC, Guzmán MC (2011) Complejo de la esclerosis tuberosa, revisión de tema y presentación de caso. Rev Asoc Colomb Dermatol 19: 76-81. Link: http://bit.ly/2lKdMvm
- Padilla F, Mendizábal R, Ayala A, Acosta R, Melo G, et al. (2012) Esclerosis tuberosa. Arch Neurocien 17: 132-137. Link: http://bit.ly/2kgr42g
- Moreno NB, Tamayo J, Moreno N, Materán M (2012) Complejo esclerosis tuberosa. A propósito de un caso. Arch Venez Pueric Pediatr 75: 121-125. Link: http://bit.ly/2khHHL0
- Dragoumi P, O'Callaghan F, Zafeiriou DI (2018) Diagnosis of tuberous sclerosis complex in the fetus. Eur J Paediatr Neurol 22: 1027-1034. Link: http://bit.ly/2lIpjex
- Mi CR, Wang H, Jiang H, Sun RP, Wang GX (2014) Mutation screening of TSC1 and TSC2 genes in Chinese Han children with tuberous sclerosis complex. Genetics Molecul Res 13: 2102-2106. Link: http://bit.ly/2khIifI
- Dill P, Liang N, Pende M (2015) New insights into the pathophysiology of the tuberous sclerosis complex: Crosstalk of mTOR - and hippo -YAP pathways in cell growth. Rare Dis 3: e1016701. Link: http://bit.ly/2kGjgHe
- Rosner M, Hanneder M, Siegel N, Valli A, Hengstschläger M (2008) The tuberous sclerosis gene products hamartin and tuberin are multifunctional proteins with a wide spectrum of interacting partners. Mutat Res 658: 234-246. Link: http://bit.ly/2kd7X9b
- Ebrahimi-Fakhari D, Meyer S, Vogt T, Pföhler C, Müller CSL, et al. (2017) Dermatological manifestations of tuberous sclerosis complex (TSC). J Dtsch Dermatol Ges 15: 695-700. Link: http://bit.ly/2lRBDcg
- Lam HC, Nijmeh J, Henske EP (2017) New developments in the genetics and pathogenesis of tumours in tuberous sclerosis complex. J Pathol 241: 219-225. Link: http://bit.ly/2lQ2TIa
- Cheng T (2012) Tuberous sclerosis complex: an update. Hong Kong J Dermatol Venereol 20: 61-67.
- Narayanan V (2003) Tuberous Sclerosis Complex: Genetics to Pathogenesis. Pediatr Neurol 29: 404-409. Link: http://bit.ly/2kb9SLq
- Curatolo P, Bombardieri R, Jozw S (2008) Tuberous sclerosis. Seminar 372: 657-668.
- Au KS, Williams AT, Roach ES, Batchelor L, Sparagana SP, et al. (2007) Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genet Med 9: 88-100. Link: http://bit.ly/2m7Dbix
- Randle SC (2017) Tuberous Sclerosis Complex: A Review. Pediatr Ann 46: e166-e171. Link: http://bit.ly/2kGXVgS
- Falsafi P, Taghavi-Zenouz A, Khorshidi-Khiyavi R, Nezami N, Asghari M (2015) A Case of Tuberous Sclerosis Without Multiorgan Involvement. Glob J Health Sci 7: 124-131. Link: http://bit.ly/2lPzoqg
- Fernandez M, Boixeda P, Anaya MJ, Belmar P, Jaén P (2009) Esclerosis tuberosa. Hallazgos clínicos en 67 pacientes. Actas Dermosifiliogr 100: 596-601. Link: http://bit.ly/2meljCT
- Fernández O, Gómez A, Sardiñaz N (1999) Esclerosis tuberosa. Revisión. Revista Cubana Pediatría 71: 160-167.
- Lizárraga SL, Zárate P, Bobadilla A, Melgoza ME (2010) Rabdomiomas intracardiacos múltiples en un neonato con esclerosis tuberosa. Informe de un caso. Acta Pediátr d Méx 31: 153-157. Link: http://bit.ly/2k8KINs
- Orlova KA, Crino PB (2010) The tuberous sclerosis complex. Ann N Y Acad Sci 1184: 87-105. Link: http://bit.ly/2lPB1Eo
- Hinton RB, Prakash A, Romp RL, Krueger DA, Knilans TK (2014) Cardiovascular Manifestations of Tuberous Sclerosis Complex and Summary of the Revised Diagnostic Criteria and Surveillance and Management Recommendations from the International Tuberous Sclerosis Consensus Group. J Am Heart Assoc 3: 1-11. Link: http://bit.ly/2kcA5Jx
- Jiménez S, Benito F, Sanchez C (2000) Rabdomiomas cardíacos en la esclerosis tuberosa: manifestaciones clínicas y evolución de 18 casos diagnosticados en la infancia. An Esp Pediatr 52: 36-40. Link: http://bit.ly/2kIcTmC
- Northrup H, Krueger DA (2013) Tuberous Sclerosis Complex Diagnostic Criteria Update: Recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 49: 243-254. Link: http://bit.ly/2khKJim
- Krueger DA, Northrup H (2013) Tuberous Sclerosis Complex Surveillance and Management: Recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 49: 255-265. Link: http://bit.ly/2khL84m
- (2011) Tuberous Sclerosis aliance, Diagnosis, screening and clinical care of individuals with tuberous sclerosis complex.
- Rabito MJ, Kaye AD (2014) Tuberous Sclerosis Complex: Perioperative Considerations. Ochsner J 14: 229-239. Link: http://bit.ly/2lREguE
- Garcia-Penas JJ (2018) Epilepsy, cognition and ketogenic diet. Rev Neurol 66: S71-S75. Link: http://bit.ly/2kgyGBV
- Park S, Lee EJ, Eom S, Kang HC, Lee JS, et al. (2017) Ketogenic diet for the management of epilepsy associated with tuberous sclerosis complex in children. J Epilepsy Res 7: 45-49. Link: http://bit.ly/2kIkq4T
- Palavra F, Robalo C, Reis F (2017) Recent advances and challenges of mTOR inhibitors use in the treatment of patients with tuberous sclerosis complex. Oxid Med Cell Longev 2017. Link: http://bit.ly/2kigfNe
- Evans LT, Morse R, Roberts DW (2012) Epilepsy surgery in tuberous sclerosis: a review. Neurosurg Focus 32: 1-6. Link: http://bit.ly/2mdrBCJ
- De Vries PJ, Belousova E, Benedik MP, Carter T, Cottin V, et al. (2018) TSC-associated neuropsychiatric disorders (TAND): findings from tehe TOSCA natural history study. Orphanet J Rare Dis 13: 157. Link: http://bit.ly/2m5uvJw
- Cubero CC, Moguel BV, Gil FMA, Vega JLA (2016) Asociación lupus eritematoso sistémico y esclerosis tuberosa, un caso. Reumatol Clin 12: 219-222. Link: http://bit.ly/2lLo3Y4
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
