Scientific J Genet Gene Ther
Caveolin-1 in renal disease
Sourabh Chand*
Cite this as
Chand S (2018) Caveolin-1 in renal disease. Scientific J Genet Gene Ther 4(1): 007-014. DOI: 10.17352/sjggt.000016Caveolin-1 is the essential structural formation for lipid raft formation. It has been ascribed to several disease processes in humans due to its ubiquitous distribution. Patients with chronic kidney disease suffer great morbidity and mortality where manipulation of caveolin-1 could lead to new potential therapeutic targets in this patient group. This review highlights caveolin-1 structure, signalling and provides examples of studies of caveolin-1 single nucleotide polymorphism in chronic kidney disease.
Introduction
Chronic kidney disease (CKD) is a major global public health issue that affects estimates of 10-16% of the general population in developed countries leading to premature morbidity and mortality [1]. In 2009-2010, the economic burden of chronic kidney disease (CKD) on the English National Health Service (NHS) was an estimated £1.45 billion (equivalent to 1.3% of all NHS spending that year), with over half of the costs related to the 2% of the CKD population with end-stage renal disease (ESRD) requiring renal replacement therapy (RRT) [2]. The resultant effect of the CKD burden is an excess in length of stay in hospitals, hospital associated infections as well as an excess of 7000 cerebral vascular events and 12000 myocardial infarctions compared to age/gender matched controls [2]. Thus, it remains paramount to identify individuals with CKD at the earliest time-point in order to instigate therapy to prevent the progression to ESRD.
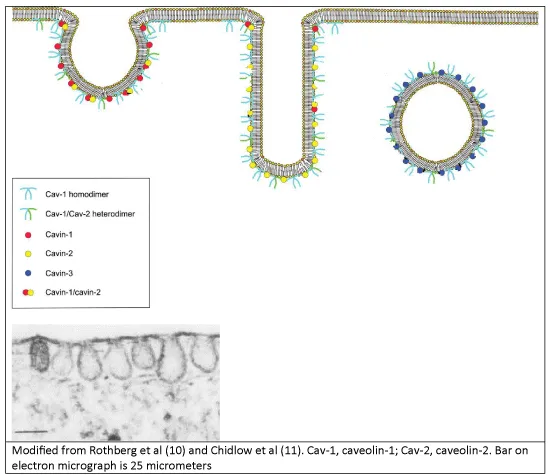
Caveolae are sub-class of non-clathrin coated lipid rafts that were morphologically first identified in 1953 via transmission electron microscopy and appear as little cave-like invaginations of the plasma membrane of 50-100 nanometres in size [3]. In 1955, Yamada first described caveolae existing in the renal mouse glomerular capillary endothelium [4]. Caveolae are ubiquitously distributed though predominately found in endothelial cells, epithelial cells, striated and smooth muscle cells, fibroblasts and type 1 pneumocytes, with almost twenty percent of the total plasma membrane of adipocytes being occupied by caveolae [5,6]. There are three main isoforms of the caveolin protein, with caveolin-1 (CAV1) being essential for the formation of caveolae. In 1999, caveolin-2 was described as 20-kDa protein that co-localises and is dependent on CAV1 to form hetero-dimers [7] and caveolae membrane localisation to the basolateral surface of epithelial cells, whilst caveolae with CAV1 alone, are not seen on the apical surface [8]. Caveolin-3 has a similar morphology to caveolin-1, and is mostly found in cardiac, smooth and skeletal myocytes [9].
Caveolae are largely composed of cholesterol with concentrated glycosphingolipids and sphingomyelin relative to the plasma membrane distribution, with CAV1 required for its structural stability. As shown in figure 1, caveolae can form either the classical omega or little cave, enlongated (for channel formation) or a direct vesicle formation mostly at the basolateral surface [10]. This depends on the proteins called cavins whose abundance changes the mobility of CAV1 and thus structural integrity of the caveolar structure allowing the structure and its contents to be endocytosed. If the ratio of cavin-1 to cavin-2 is higher, then the caveolae omega shape is favoured; if cavin-2 expressed levels higher than cavin-1, then the elongated shape is formed whilst cavin-3 expression directs vesicle formation [11]. Depletion of cholesterol results in decreased CAV1 expression that can also lead to destabilisation of the caveolae structure to become mobile from the plasma membrane.
Caveolin-1 is a protein that remains intracellular, formed via the endoplasmic reticulum (ER) system and transported from the Golgi apparatus to the plasma membrane. CAV1 exit from the Golgi apparatus is accelerated by increasing cholesterol and inhibited after glycosphingolipid depletion [12]. There are two isoforms of CAV1, alpha of 178 amino acid length and beta which is 31 amino acid lengths shorter, with the former isoform having a higher affinity for the plasma membrane. The N’ terminal and C’ termini face the cytoplasm, after tyrosine phosphorylation and palmitoylation respectively. Between the termini, there is a hairpin structure hydrophobic domain of 32 amino acids (residues 102-134) that inserts to plasma membrane and is involved in hetero-oligomerization of CAV1 with caveolin-2 [13]. Residues 82-101 and 135-150 flank this region, with the latter termed C’ membrane attachment domain that contains a cis-Golgi targeting sequence. The N’ membrane attachment domain or caveolin scaffolding domain (residue 82-101) is integral in membrane localisation and anchoring of various proteins within caveolae and regulation (both inhibition and enhancement) of their signalling activity [5]. Another important site is the tyrosine-14 residue (Y14), which requires phosphorylation for caveolar endocytosis and is important in cell adhesion.
Caveolin-1 gene
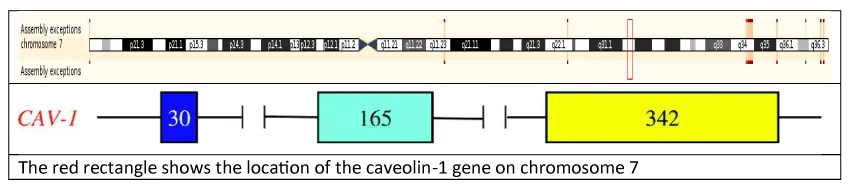
The human caveolin-1 gene is located on the long arm of chromosome 7, at genomic locus 7q31.1 (7:116540796) as shown in figure 2. It consists of 3 exons (30, 165 and 342 base pair in length respectively) and 2 introns (1.5 and 32 kilobases in length) spaced in-between. The first exons contain CpG islands that via methylation are thought to be the main part of CAV1 gene expression in cancer cell lines [14]. The third exon harbours the functional oligomerisation, scaffolding, transmembrane and C’ membrane attachment domains that are highly conserved across species [15].
Caveolin-1 signalling
There are several roles of caveolae and CAV1 which are integral to cellular function. The most common involves vesicular transport of macromolecules (such as albumin) via transcytosis from the luminal side of the capillary endothelium to the interstitial space via membrane-bound vesicles. The accumulation of gold-labelled albumin in the interstitial space is not seen in the knockout CAV1 mouse by transmission electron microscopy as compared to the wild-type mouse [16]. Endocytosis is the second example of vesicular membrane trafficking by caveolae. Caveolae share similar vesicle docking and fusion molecules (soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) and dynamin) as seen in the traditional clathrin-mediated endocytosis. Pathogens such as Simian virus 40 and the cholera toxin utilise collections of caveolae for internalisation into the cell, forming with a distinct endosomal compartment with a neutral pH called a caveosome, for delivery to the ER and Golgi apparatus with recycling of CAV1 to the plasma membrane [5,17].
CAV1 and caveolae are also integral to cellular signalling and signal transduction by compartmentalising receptors upon ligand binding, acting as a chaperone of signalling molecules and ligand bound receptors for delivery to the cell nucleus as well as concentrating such events in the confines of a specific subcellular environment [5]. Receptors such as the epidermal growth factor receptor (EGFR) and transforming growth factor beta (TGFβ) receptor are internalised by caveolae upon ligand binding after phosphorylation of Y14 part of CAV1 [18]. This internalisation is dependent on Src kinases and protein kinase C [19]. Golgi derived CAV1 via SNARE protein syntaxin-6 is also essential for plasma membrane delivery of receptors angiotensin II type 1, insulin and the stretch activated channel short transient receptor potential channel-1 [12].
In the majority, CAV1 effects are inhibitory upon cellular signalling, acting to degrade receptors except in the case of the insulin receptors. The main outcomes of CAV1 signal association are pro-apoptotic and anti-fibrotic. The most commonly described signalling CAV1 associations are with endothelial nitric oxide synthase (eNOS) and TGFβ pathways. CAV1 via its CSD binds eNOS resulting in its inactivity and can act as a reservoir of inactivated eNOS. The influx of calcium leads to more calmodulin recruitment and binding to the eNOS enzyme leading to its release from bound CAV1 and thus restoration of electron flow from NADPH to its reductase domain flavins and then to the heme moiety of eNOS, generating the vasodilator nitric oxide (NO) from L-arginine. The generation of NO leads to a dissociation of CAV1 scaffold, leading to termination of signal transduction. However, if the substrate L-arginine becomes limited, eNOS functions in an uncoupled mode, leading to the electron transfer to the heme moiety to react with oxygen to form reactive oxygen species (ROS) [20].
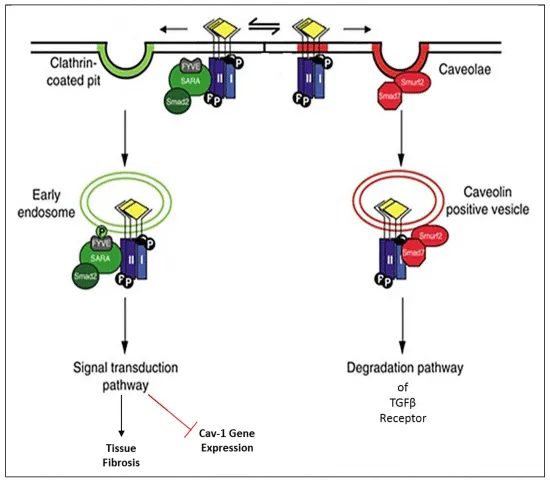
ROS releases TGFβ-1 from its latency-associated peptide and latent TGFβ-binding protein to become activated [21]. Upon this ligand binding, TGFβ type 1 receptor is phosphorylated after binding with TGFβ type II receptor to form a serine/threonine kinase heterotetrameric complex (Figure 3). If this complex is internalised by the early-endosome antigen 1 non-lipid/clathrin raft pathway, this phosphorylates the SARA/Smad 2/3 complex downstream to induce a conformational change that results in heteromerization of Smad 4 and translocation to the nucleus to regulate transcription of pro-fibrotic target genes as well as the downregulation of CAV1 and Smad 7 [22]. However, if the TGFβ receptor I/II complex is internalised by caveolae, then SMURF/Smad 7 is recruited and exerts a negative effect upon TGFβ signalling by proteasomal degradation and ubiquitination of the TGFβ receptor complex in CAV1 positive vesicles. In addition, CAV1 diminishes Smad 2 phosphorylation, disrupts its interaction with Smad 4, and prevents Smad 2 translocation to the nucleus, further reducing TGFβ signalling [22].
Other signalling events associated with caveolae endocytosis involve fibronectin degradation and integrin internalisation [23,24]. The detachment of fibroblasts triggers ganglioside GM1 internalisation via CAV1 and its Y14 phosphorylation; GM1 internalisation leads to Rac1 loss from the plasma membrane and its reduced activation [12]. Thus not only is CAV1 important in compartmentalising signal transduction in caveolae by concentrating and localising signalling molecules by acting as docking points for numerous cell surface receptors after ligand binding, but also regulates cell adhesion, cell migration via β1-integrins, extracellular matrix (ECM) interactions and acts as flow sensors in endothelial cells and regulates stretch-induced cell cycle progression [12,24]. CAV1 response to chronic shear stress is to increase its levels with redistribution from the Golgi complex to the plasma membrane and formation of caveolae [25]. This leads to increased mechanosensitivity with activation of signalling pathways such as eNOS and mitogen-activated protein kinase (MAPK). With Cav1-/- mice, they exhibit defects in chronic flow-dependent remodelling of blood vessels [26]. Upon stretch, cell cycle progression is inhibited with CAV1 downregulation or in Cav1-/- smooth muscle cells through pathways including PI3 kinase-AKT/protein kinase B, MAPK ERK, c-Src and integrins [27].
Caveolin-1 in renal adverse outcomes
Caveolin-1 in renal fibrosis: As kidney function deteriorates in CKD, more interstitial fibrosis is found amongst patients irrespective of their underlying cause of CKD. CAV1 has been an attractive protein to investigate in the context of organ fibrosis as suggested with its effects described in the previous section and the following section summarises renal fibrotic CAV1 research [28].
CAV1 interaction with TGFβ in fibrosis is a common pathway by which renal and non-renal chronic disease progresses and thus has been extensively investigated. Li et al. used primary murine pulmonary endothelial cells that were capable of obtaining a myofibroblast phenotype after TGFβ-1 stimulation, inducing αSMA expression from wild-type cells. Cav1-/- cells produced very high spontaneous levels of αSMA that was corrected upon CAV1 functional restoration using its CSD peptide; thus CAV1 regulates endothelial-to-mesenchymal transition in tissue fibrosis [29]. Ito et al. [30], showed that adding hyaluronan with its receptor CD44 to immortalised renal proximal tubular epithelial cells (HK2) led to an increase of lipid raft internalisation of TGFβ via MAP kinase from the non-lipid pathway predominance. The authors co-cultured HK2 cells with cells transfected with a Smad responsive promoter and blocked several components (e.g. CD44 and its interaction with hyaluronan) along this pathway to confirm their finding. As the authors state, this conflicts with previous work with metastatic breast tumour cells that suggest hyaluronan promoted the non-lipid pathway, and thus the finding may be dependent on specific tissue microenvironments. Whilst Zhang et al. [31], have shown stimulation with IL-6 led to more non-lipid raft pathway dominance for the TGFβ receptor in HK2 cells and a decrease in the association of the IL-6 receptor to CAV1 upon co-immunoprecipitation. This was after the observation that IL-6 is only expressed on proximal tubular epithelial cells at times of renal disease. However despite the affinity labelling findings, it was noted that stimulation with IL-6 and TGFβ-1 did not lead to an increase in Smad 2 or 3 or 7 protein expression, but only the luciferase assay suggested more Smad activity.
As well as TGF, there have been many other signalling events associated with CAV1 in renal fibrosis. Peng et al [32], showed a Src dependent phosphorylation of the CAV1 Y14 site occurred with RhoA activation and formation of ROS when they mechanically stretched mesangial cells via a cyclic vacuum as a model of intraglomerular hypertension leading to glomerulosclerosis. In the Cav1 knockout mouse, there was no RhoA or ROS activation but this function was promptly restored upon restoration of Cav1 [33].
As another model of hypertension, Bocanegra et al. [34], investigated the protective profile of losartan upon proximal tubular cells from spontaneously hypertensive rats, whose CAV1 expression was significantly reduced compared to control. Losartan (an angiotensin II type 1 receptor antagonist) was noted to reduce ROS through Nox4 downregulation and NADPH inactivation, through increased levels of CAV1 and co-localisation with Heat shock protein 70. However two questions remain: was this just a by-product of the blood pressure lowering ability of losartan and why none of these effects were seen in the control group? The editorial comment focussed on the mechanisms behind hypertension and ROS production causing reduced renal function and structural damage; this was mainly to inappropriate activation of intrarenal angiotensin II within the proximal tubules due to its translocation of angiotensin II type 1 receptor (AT1) from the plasma membrane to CAV1 rich domains. Here, CAV1 acts as a molecular chaperone to AT1, providing a platform for redox signalling events through NADPH oxidase dependent production of ROS via Nox4 [35].
Genetically obese Zucker rats on a casein diet have also been shown to have reduced eNOS and raised CAV1 in renal structural damaged kidneys as compared to those on a soy diet, but again, CAV1 is known to fluctuate with cholesterol levels making it difficult to interpret the findings [36]. From Valles et al. [37], ureteric obstruction leads to an early rapid intrarenal angiotensin II rise that leads to an increase in extracellular matrix protein production and oxidative stress damage causing fibrosis. Nitric oxide can ameliorate interstitial fibrosis and in loss of eNOS leads to marked tubulointerstitial fibrosis in the inducible NOS knockout mouse. CAV1 is expressed in vascular endothelium, smooth muscle and epithelial cells of the proximal, distal and convoluted tubules and ducts. CAV1 also leads to apoptosis via inhibition of p42/44 MAP kinase signalling. Thus the authors wished to look at the role of CAV1 expression in congenital unilateral ureteropelvic junction obstruction with grade IV hydronephrosis on imaging requiring surgical intervention after vesico-ureteral reflux was excluded. The 19 children had ‘normal renal function for their age’, were on no medication and were normotensive. The scorers of interstitial volume and CAV1 staining were blinded. There were two groups with group 1 split into subset A with obstruction <1yr, subset B with obstruction >1yr, and group 2 had significant renal impairment compared to group 1 (99mTc-DPTA renal scan expression of kidney filtration rate 28.8±2% to 39.7±2.1% respectively). The results showed group 2 CAV1 was present in the proximal tubule unlike control and group 1, as well as co-localising with the AT1 receptor. Increased CAV1 expression was confirmed on Western Blotting for protein transcription from group 2’s urine. eNOS was low in group2 (p<0.01). Unfortunately the control was based on children with renal cell carcinoma where it is known that CAV1 has expression can be altered and may thus not act as a true control.
Further to the above human study suggesting increased CAV1 expression in the more chronic and fibrotic group in children, histological increased glomerular expression of CAV1 in Japanese patients has been identified in diseases that target the glomerulus such as diabetic nephropathy, membranous nephropathy and focal segmental glomerulosclerosis [38]. It was also noted that CAV1 expression was also reduced in glomerular endothelial cells with the use of steroids. In diabetic nephropathy, murine Cav1 knockout led to a worse glomerulosclerosis and albuminuria in the streptozotocin model of type 1 diabetic nephropathy, though there is limited tubulointerstitial fibrosis in this model [39]. CAV1 increased expression was also noted in a streptozotocin rat model of diabetic nephropathy with increased VEGF receptor 2 (VEGFR2)/CAV1 association in vivo [40]. In vitro, primary rat mesangial cells after stimulation with VEGF, caused more CAV1/VEGFR2, CAV1/Src expression and fibronectin upregulation via RhoA activation. Transfection of mesangial cells with an overexpression of non-phosphorylatable CAV1 Y14 prevented VEGF-induced RhoA activation and fibronectin upregulation. Another model of nodular glomerulosclerosis utilises human urinary free light chains from patients suffering from light chain deposition disease injecting to wild-type and Cav1 knockout rats’ tail veins to induce nodular glomerulosclerosis. In the Cav1 knockout, there was increased nodular glomerulosclerosis and increased mesangial matrix production, however there was a mix of gender used that may have influenced the results [41].
Other human studies have focussed on CAV1 in renal transplantation. Yamamoto et al. [42], have previously shown that de novo caveolae formation occurs in transplant glomerulopathy in glomerular endothelial cells and now investigate CAV1 in chronic active antibody mediated rejection and thus transplant capillaropathy, where they found CAV1 to be associated with peritubular capillary endothelia that is not normally present in healthy kidney. Pontrelli et al. [43], also investigated chronic allograft nephropathy secondary to immune system activation where CD40 is upregulated in acute rejection in proximal tubular epithelial cells. This led to increased Lyn phosphorylation and thus NFҡB activation. Lyn phosphorylation is strictly associated with CAV1 and inhibiting Lyn blocked the profibrotic induction of PAI-1.
Park et al. [44], have used an unilateral ureteric obstruction (UUO) model in FVB/N mice to investigate the surge of stem cells that occurs upon 10 days after ligation of the left ureter. In the Cav1 knockout, the mesenchymal stem cell post obstructive surge was blunted significantly which led to no regeneration of the parenchyma and marked fibrosis as measured by Sirius red staining. Indeed some of the surge could be from the response of the resident kidney stem cells. In this model, the FVB/N wild-type mice are a different strain to the Cav1 knockout mice and the control kidney was the contralateral kidney of each mouse. These factors could affect the interpretation of their findings as different mouse strains have varying susceptibility to fibrosis and the contralateral compensation after ligation of the ureter could be different.
Chand et al. [45], also investigated the UUO model at day 3 and day 14 with sham operated mice being the same age, strain and gender as mice undergoing UUO as their Cav1 knockout mice. They showed that there was a more profound fibrosis at day 14 of the UUO model with the Cav1 knockout mice than their wild-type counterparts, but at day 3 the wild-type had more fibrosis. The main difference was the abundance of F4/80 positive stained cells on confocal microscopy of frozen kidney sections in the wild-type compared to the Cav1 knockout mice.
In summary, there appears to be negative effect of CAV1 expression leading to a worse renal phenotype especially in glomerular disease. However, this is in opposition to the non-renal literature where the CAV1 reduction in patient samples or knockout of caveolin-1 in vitro has been found to lead to a more fibrotic phenotype. This may be due to the organ and microenvironment studied such as in bleomycin induced lung fibrosis [46], idiopathic pulmonary fibrosis [47], scleroderma/systemic sclerosis [48], cardiac fibrosis [49], and keloid scars [50], which involve more TGFβ dependent processes that are reliant on the expression of CAV1 in fibroblasts.
Pleiotropic effects of caveolin-1 in adverse renal outcomes
As well as tubulointerstitial fibrosis, there are common adverse outcomes experienced by patients with CKD as their renal disease progresses. Due its ubiquitous nature of distribution, CAV1 has been associated with many of these outcomes.
Infection: In patients suffering from scleroderma associated lung disease, their bronchoalveolar lavage revealed activated monocytes and polymorphonuclear cells; these patients’ monocytes, neutrophils and T cells had a reduced expression of CAV1 [50]. In mice exposed to lipopolysaccharide challenge, the deletion of Cav1 led to a decreased expression of CD14 and CD36 during macrophage differentiation and suppressed phagocytic ability and impaired bacterial clearance [51]. Cav1 knockout mice also had an increased mortality with induced Pseudomonas aeruginosa and Klebsiella pneumoniae sepsis [52,53]. In cystic fibrosis, effective internalisation of pathogens such as Pseudomonas aeruginosa is required for an appropriate immune response for its clearance; its internalisation is reliant on CAV1 in type 1 pneumocytes and bladder epithelium [54]. CAV1 interaction with protein kinase Cα upon activation and calcium release is essential for its translocation to caveolae in order for production of infectious enveloped human cytomegalovirus particles in fibroblasts [55]. Infection with polyomavirus viraemia can cause BK nephropathy in renal transplant recipients. Moriyama et al. in vitro data shows this viral entry in human proximal tubular epithelial cells requires co-localisation with CAV1 for caveolae entry into the cells [56].
Cardiovascular disease: CAV1 has been associated with an inflammatory macrophage phenotype that promotes atherosclerosis by the production of foam cells [57]. Schwencke et al. have found reduced CAV1 expression in VSMC of human atheroma [58]. CAV1 also binds eNOS in an inactive state and with an influx of calcium, its release thus affecting vascular function [59]. In the Cav1 knockout mouse, cardiac hypertrophy occurs despite the high presence of caveolin-3, in which the latter is thought to be the predominant isoform of caveolin in the heart [60].
Malignancy: CAV1 may have a tumour suppressive role depending on the cancer type. CAV1 has been shown to promote apoptosis and in Cav1 knockout mice, hyperactivation of the p42/44 MAP kinase cascade and cyclin D1 upregulation in breast cancer models lead to cancer progression and metastases [61]. CAV1 has been considered as a prognostic marker in several cancers [62]. However, in prostate cancer, CAV1 upregulation has been associated with progression of the malignancy [63], highlighting CAV1 altered function depending on the organ microenvironment studied.
Caveolin-1 single nucleotide polymorphism in renal disease: Testa et al. found that there was a significant independent and interaction association between CAV1 rs4730751 and eNOS rs1799983 genotype and increased carotid arterial intima thickness (vascular hypertrophy) and cross-sectional area across the common carotid artery (arterial remodelling) [64]. It is not clear if these associations occur in non-dialysis CKD or just a phenomenon seen in haemodialysis patients. eNOS is bound to CAV1 requiring a calcium influx for its release from CAV1 in order to become activated.
Importantly, as CKD becomes more advanced the dominant vascular lesion is arteriosclerosis and associated vascular stiffness, rather than atheromatous disease as seen in the general population [65]. Aortic pulse wave velocity (aPWV) is the gold standard method for measuring arterial stiffness and has been consistently associated with all-cause and cardiovascular mortality in multiple conditions including CKD [66,67]. Independent of known clinical variables that influence aPWV in multivariate analysis, Chand et al. found that CAV1 rs4730751 CC genotype is associated with lower arterial stiffness in patients with early and late stage non-dialysis CKD [68]. In vascular endothelium CAV1 interacts with eNOS such that reduced CAV1 increases eNOS activity which may have a deleterious effect on endothelial health and arterial stiffness, due to “uncoupling” of eNOS which leads to the generation of superoxide anion radicals [69], in oxidative stress characteristic of CKD [70]. Similarly, caveolin-1 deficient aortic smooth muscle cells have been shown to be pro-arteriosclerotic with increased neointimal hyperplasia, cell proliferation and migration [71]. These observations may underlie the findings of the current study. Conversely, lower levels of CAV1 in macrophages are associated with an anti-inflammatory phenotype, reduced foam cell formation and therefore protection from atheroma [57]. Interestingly, these contrasting functions of CAV1 in endothelium (“anti-arteriosclerotic”) and macrophages (“pro-atheromatous”) may consolidate the findings of the current study whereby CC genotype associates with a reduced aPWV (“anti-arteriosclerotic”) and the findings of Testa et al. [64], who showed an association between CC genotype and increased carotid arterial intima media thickness (a measure of atheroma rather than arteriosclerosis [72].
Anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitis represent a group of primary autoimmune disorders that are systemic, mainly involving small to medium sized vessels. Despite success in improving patient life expectancy, there remains high mortality at 5 years (up to 28%) and significant morbidity associated with complications of the disease and its treatment such as infection, cardiovascular disease, malignancy and progression of kidney disease. These complications mirror potential CAV1 effects, and indeed, the CC genotype of CAV1 rs4739751 was associated with a better outcome in this group of patients [73].
Donor CAV1 AA rs4739751 genotype of renal transplants are associated with worsening renal allograft function, survival as well as excess fibrosis upon histology in cohorts from Birmingham [74], Belfast [74], and France [75].
Conclusion
CAV1 pleotropic effects and ubiquitous distribution makes it an attractive therapeutic target in many human diseases in particular with patients in CKD, either to reduce related morbidity and mortality, as a biomarker for identification of high risk individuals, but also its direct manipulation in renal fibrosis.
- Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, et al. (2007) Prevalence of chronic kidney disease in the United States. Jama 298: 2038-2047. Link: https://tinyurl.com/ybej3mkq
- Kerr M, Bray B, Medcalf J, O'Donoghue DJ, Matthews B (2012) Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant 3: 73-80. Link: https://tinyurl.com/y7v7mlgb
- Palade GE (1953) Fine Structure of Blood Capillaries. J Appl Phys 24: 1424-. Link: https://tinyurl.com/yaxkzuv6
- Yamada E (1955) The fine structure of the renal glomerulus of the mouse. J Biophys Biochem Cytol 1: 551-566. Link: https://tinyurl.com/ybeffaoq
- Cohen AW, Hnasko R, Schubert W, Lisanti MP (2004) Role of caveolae and caveolins in health and disease. Physiol Rev 84: 1341-1379. Link: https://tinyurl.com/yajh8g76
- Fan JY, Carpentier JL, van Obberghen E, Grunfeld C, Gorden P, et al. (1983) Morphological changes of the 3T3-L1 fibroblast plasma membrane upon differentiation to the adipocyte form. J Cell Sci 61: 219-230. Link: https://tinyurl.com/yad4r2rw
- Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, et al. (1997) Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem 272: 29337-2946. Link: https://tinyurl.com/yadnrdp4
- Scheiffele P, Verkade P, Fra AM, Virta H, Simons K, et al. (1998) Caveolin-1 and -2 in the exocytic pathway of MDCK cells. J Cell Biol 140: 795-806. Link: https://tinyurl.com/y7kjwsav
- Tang Z, Scherer PE, Okamoto T, Song K, Chu C, et al. (1996) Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem 271: 2255-2261. Link: https://tinyurl.com/yd3rlxlu
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, et al. (1992) Caveolin, a protein component of caveolae membrane coats. Cell 68: 673-682. Link: https://tinyurl.com/yae2ao6h
- Chidlow JH, Sessa WC (2010) Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res 86: 219-225. Link: https://tinyurl.com/y8eahgd5
- Parton RG (2007) Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol 8: 185-194. Link: https://tinyurl.com/yc67m8az
- Tanase C (2009) CAV1 (caveolin 1, caveolae protein, 22kDA). Atlas Genet Cytogenet Oncol Haematol 13: 788-792. Link: https://tinyurl.com/y8cmcbxz
- Engelman JA, Zhang XL, Lisanti MP (1999) Sequence and detailed organization of the human caveolin-1 and -2 genes located near the D7S522 locus (7q31.1). Methylation of a CpG island in the 5' promoter region of the caveolin-1 gene in human breast cancer cell lines. FEBS Lett 448: 221-230. Link: https://tinyurl.com/yd7xkukp
- Engelman JA, Zhang X, Galbiati F, Volonte D, Sotgia F, et al. (1998) Molecular genetics of the caveolin gene family: implications for human cancers, diabetes, Alzheimer disease, and muscular dystrophy. Am J Hum Genet 63: 1578-1587. Link: https://tinyurl.com/y8pwqt7k
- Schubert W, Frank PG, Razani B, Park DS, Chow CW, et al. (2001) Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J Biol Chem 276: 48619-48622. Link: https://tinyurl.com/yahzyjrn
- Norkin LC, Anderson HA, Wolfrom SA, Oppenheim A (2002) Caveolar endocytosis of simian virus 40 is followed by brefeldin A-sensitive transport to the endoplasmic reticulum, where the virus disassembles. J Virol 76: 5156-5166. Link: https://tinyurl.com/ycds7ejm
- del Pozo MA, Balasubramanian N, Alderson NB, Kiosses WB, Grande-Garcia A, et al. (2005) Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol 7: 901-908. Link: https://tinyurl.com/yawwgn2w
- Sharma DK, Brown JC, Choudhury A, Peterson TE, Holicky E, et al. (2004) Selective stimulation of caveolar endocytosis by glycosphingolipids and cholesterol. Mol Biol Cell 15: 3114-3122. Link: https://tinyurl.com/y75lbazb
- Goligorsky MS, Li H, Brodsky S, Chen J (2002) Relationships between caveolae and eNOS: everything in proximity and the proximity of everything. Am J Physiol Renal Physiol 283: 1-10. Link: https://tinyurl.com/y7avdjob
- Meng XM, Tang PM, Li J, Lan HY (2015) TGF-beta/Smad signaling in renal fibrosis. Front Physiol 6: 82. Link: https://tinyurl.com/y8526uxx
- Razani B, Zhang XL, Bitzer M, von Gersdorff G, Bottinger EP, et al. (2001) Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J Biol Chem 276: 6727-6738. Link: https://tinyurl.com/y9nfvr55
- Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, et al. (2008) Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell 15: 209-219. Link: https://tinyurl.com/y9fcvxgx
- Shi F, Sottile J (2008) Caveolin-1-dependent beta1 integrin endocytosis is a critical regulator of fibronectin turnover. J Cell Sci 121: 2360-2371. Link: https://tinyurl.com/yd4sd8kt
- Rizzo V, Morton C, DePaola N, Schnitzer JE, Davies PF (2003) Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro. Am J Physiol Heart Circ Physiol 285: 1720-1729. Link: https://tinyurl.com/ybqszn54
- Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, et al. (2006) Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest 116: 1284-1291. Link: https://tinyurl.com/y8llvpw9
- Sedding DG, Hermsen J, Seay U, Eickelberg O, Kummer W, et al. (2005) Caveolin-1 facilitates mechanosensitive protein kinase B (Akt) signaling in vitro and in vivo. Circ Res 96: 635-642. Link: https://tinyurl.com/y79uc7sm
- Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL (2003) Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol 5: 410-421. Link: https://tinyurl.com/y9frhfxx
- Li Z, Wermuth PJ, Benn BS, Lisanti MP, Jimenez SA (2013) Caveolin-1 deficiency induces spontaneous endothelial-to-mesenchymal transition in murine pulmonary endothelial cells in vitro. Am J Pathol 182: 325-331. Link: https://tinyurl.com/yaky3n3n
- Ito T, Williams JD, Fraser DJ, Phillips AO (2004) Hyaluronan regulates transforming growth factor-beta1 receptor compartmentalization. J Biol Chem 279: 25326-25332. Link: 'https://tinyurl.com/yd4ffhh2
- Zhang XL, Topley N, Ito T, Phillips A (2005) Interleukin-6 regulation of transforming growth factor (TGF)-beta receptor compartmentalization and turnover enhances TGF-beta1 signaling. J Biol Chem 280: 12239-12245. Link: https://tinyurl.com/y77rencp
- Peng F, Wu D, Ingram AJ, Zhang B, Gao B, Krepinsky JC (2007) RhoA activation in mesangial cells by mechanical strain depends on caveolae and caveolin-1 interaction. J Am Soc Nephrol 18: 189-198. Link: https://tinyurl.com/yb7jsvpq
- Zhang Y, Peng F, Gao B, Ingram AJ, Krepinsky JC (2010) Mechanical strain-induced RhoA activation requires NADPH oxidase-mediated ROS generation in caveolae. Antioxid Redox Signal 13: 959-973. Link: https://tinyurl.com/yaaqra2u
- Bocanegra V, Manucha W, Pena MR, Cacciamani V, Valles PG (2010) Caveolin-1 and Hsp70 interaction in microdissected proximal tubules from spontaneously hypertensive rats as an effect of Losartan. J Hypertens 28: 143-155. Link: https://tinyurl.com/y9al6oxd
- Nishiyama A, Hitomi H (2010) Role of caveolin and heat shock protein 70 interaction in the antioxidative effects of an angiotensin II type 1 receptor blocker in spontaneously hypertensive rats proximal tubules. J Hypertens 28(1): 9-12. Link: https://tinyurl.com/ybk98ndv
- Trujillo J, Ramirez V, Perez J, Torre-Villalvazo I, Torres N, et al. (2005) Renal protection by a soy diet in obese Zucker rats is associated with restoration of nitric oxide generation. Am J Physiol Renal Physiol 288: 108-116. Link: https://tinyurl.com/ycj8weq4
- Valles PG, Manucha W, Carrizo L, Vega Perugorria J, Seltzer A, et al. (2007) Renal caveolin-1 expression in children with unilateral ureteropelvic junction obstruction. Pediatr Nephrol 22: 237-248. Link: https://tinyurl.com/y9lmuntd
- Moriyama T, Tsuruta Y, Shimizu A, Itabashi M, Takei T, et al. (2011) The significance of caveolae in the glomeruli in glomerular disease. J Clin Pathol 64: 504-509. Link: https://tinyurl.com/ydxytkd7
- Guan TH, Chen G, Gao B, Janssen MR, Uttarwar L, et al. (2013) Caveolin-1 deficiency protects against mesangial matrix expansion in a mouse model of type 1 diabetic nephropathy. Diabetologia 56: 2068-2077. Link: https://tinyurl.com/ydhhcpvw
- Wu T, Zhang B, Ye F, Xiao Z (2013) A potential role for caveolin-1 in VEGF-induced fibronectin upregulation in mesangial cells: involvement of VEGFR2 and Src. Am J Physiol Renal Physiol 304: 820-830. Link: https://tinyurl.com/yc426rze
- Herrera GA, Turbat-Herrera EA, Teng J (2016) Animal Models of Light Chain Deposition Disease Provide a Better Understanding of Nodular Glomerulosclerosis. Nephron. Link: https://tinyurl.com/ybggqt3e
- Yamamoto I, Horita S, Takahashi T, Kobayashi A, Toki D, et al. (2008) Caveolin-1 expression is a distinct feature of chronic rejection-induced transplant capillaropathy. Am J Transplant 8: 2627-2635. Link: https://tinyurl.com/y93ff8wy
- Pontrelli P, Ursi M, Ranieri E, Capobianco C, Schena FP, et al. (2006) CD40L proinflammatory and profibrotic effects on proximal tubular epithelial cells: role of NF-kappaB and lyn. J Am Soc Nephrol 17: 627-636. Link: https://tinyurl.com/y8xpnv6m
- Park HC, Yasuda K, Ratliff B, Stoessel A, Sharkovska Y, et al. (2010) Postobstructive regeneration of kidney is derailed when surge in renal stem cells during course of unilateral ureteral obstruction is halted. Am J Physiol Renal Physiol 298: 357-364. Link: https://tinyurl.com/ybfmdbrg
- Chand S, Hazeldine J, Smith SW, Borrows R (2018) Genetic deletion of the lipid raft protein caveolin-1 leads to worsening renal fibrosis. J Clin Nephrol Ren Care 4: 037. Link: https://tinyurl.com/y9we8suq
- Tourkina E, Richard M, Oates J, Hofbauer A, Bonner M, et al. (2010) Caveolin-1 regulates leucocyte behaviour in fibrotic lung disease. Ann Rheum Dis 69: 1220-1226. Link: https://tinyurl.com/y9xe3z43
- Wang XM, Zhang Y, Kim HP, Zhou Z, Feghali-Bostwick CA, et al. (2006) Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med 203: 2895-2906. Link: https://tinyurl.com/yaub5tm8
- Del Galdo F, Sotgia F, de Almeida CJ, Jasmin JF, Musick M, et al. (2008) Decreased expression of caveolin 1 in patients with systemic sclerosis: crucial role in the pathogenesis of tissue fibrosis. Arthritis Rheum 58: 2854-2865. Link: https://tinyurl.com/ycymo8wg
- Miyasato SK, Loeffler J, Shohet R, Zhang J, Lindsey M, et al. (2011) Caveolin-1 modulates TGF-beta1 signaling in cardiac remodeling. Matrix Biol 30: 318-329. Link: https://tinyurl.com/ycmmypq7
- Zhang GY, Yu Q, Cheng T, Liao T, Nie CL, et al. (2011) Role of caveolin-1 in the pathogenesis of tissue fibrosis by keloid-derived fibroblasts in vitro. Br J Dermatol 164: 623-627. Link: https://tinyurl.com/y9x64v6r
- Tsai TH, Chen SF, Huang TY, Tzeng CF, Chiang AS, et al. (2011) Impaired Cd14 and Cd36 expression, bacterial clearance, and Toll-like receptor 4-Myd88 signaling in caveolin-1-deleted macrophages and mice. Shock 35: 92-99. Link: https://tinyurl.com/yanf9ym3
- Gadjeva M, Paradis-Bleau C, Priebe GP, Fichorova R, Pier GB (2010) Caveolin-1 modifies the immunity to Pseudomonas aeruginosa. J Immunol 184: 296-302. Link: https://tinyurl.com/y9fneylx
- Guo Q, Shen N, Yuan K, Li J, Wu H, et al. (2012) Caveolin-1 plays a critical role in host immunity against Klebsiella pneumoniae by regulating STAT5 and Akt activity. Eur J Immunol 42: 1500-1511. Link: https://tinyurl.com/y8us3qaa
- Zeitlin PL (2009) Pseudomonas aeruginosa: can studies in engineered cells tell us why isit such a problem in people with cystic fibrosis? Focus on "Cystic fibrosis transmembrane conductance regulator and caveolin-1 regulate epithelial cell internalization of Pseudomonas aeruginosa". Am J Physiol Cell Physiol 297: 235-237. Link: https://tinyurl.com/y757gvhx
- Sharon-Friling R, Shenk T (2014) Human cytomegalovirus pUL37x1-induced calcium flux activates PKCalpha, inducing altered cell shape and accumulation of cytoplasmic vesicles. Proc Natl Acad Sci U S A 111: 1140-1148. Link: https://tinyurl.com/ycz69ch9
- Moriyama T, Marquez JP, Wakatsuki T, Sorokin A (2007) Caveolar endocytosis is critical for BK virus infection of human renal proximal tubular epithelial cells. J Virol 81: 8552-8562. Link: https://tinyurl.com/y9m3ayeo
- Fu Y, Moore XL, Lee MK, Fernandez-Rojo MA, Parat MO, et al. (2012) Caveolin-1 Plays a Critical Role in the Differentiation of Monocytes into Macrophages. Arterioscler Thromb Vasc Biol. Link: https://tinyurl.com/ydhvl9db
- Schwencke C, Schmeisser A, Walter C, Wachter R, Pannach S, et al. (2005) Decreased caveolin-1 in atheroma: loss of antiproliferative control of vascular smooth muscle cells in atherosclerosis. Cardiovasc Res 68: 128-315. Link: https://tinyurl.com/ydggtltn
- Zhou X, He P (2010) Endothelial [Ca2+]i and caveolin-1 antagonistically regulate eNOS activity and microvessel permeability in rat venules. Cardiovasc Res 87: 340-347. Link: https://tinyurl.com/y8yymcbs
- Schwencke C, Braun-Dullaeus RC, Wunderlich C, Strasser RH (2006) Caveolae and caveolin in transmembrane signaling: Implications for human disease. Cardiovasc Res 70: 42-49. Link: https://tinyurl.com/yb5fl4xr
- Williams TM, Lisanti MP (2005) Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol 288: 494-506. Link: https://tinyurl.com/y8vx6zso
- Sotgia F, Martinez-Outschoorn UE, Howell A, Pestell RG, Pavlides S, et al. (2012) Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annu Rev Pathol 7: 423-467. Link: https://tinyurl.com/y96lq4xh
- Mathieu R, Klatte T, Lucca I, Mbeutcha A, Seitz C, et al. (2015) Prognostic value of Caveolin-1 in patients treated with radical prostatectomy: a multicentric validation study. BJU Int. Link: https://tinyurl.com/ybucyl8w
- Testa A, Spoto B, Sanguedolce MC, Parlongo RM, Pisano A, et al. (2012) eNOS and caveolin-1 gene polymorphisms interaction and intima media thickness: a proof of concept study in ESRD patients. Am J Hypertens 25: 103-108. Link: https://tinyurl.com/yamms5sw
- Chue CD, Townend JN, Steeds RP, Ferro CJ (2010) Arterial stiffness in chronic kidney disease: causes and consequences. Heart 96: 817-823. Link: https://tinyurl.com/ybkg7g3a
- Vlachopoulos C, Aznaouridis K, Stefanadis C (2010) Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. Journal of the American College of Cardiology 55: 1318-1327.Link: https://tinyurl.com/yc36btk2
- Baumann M, Wassertheurer S, Suttmann Y, Burkhardt K, Heemann U (2014) Aortic pulse wave velocity predicts mortality in chronic kidney disease stages 2-4. J Hypertens 32: 899-903. Link: https://tinyurl.com/yacj2dqy
- Chand S, Edwards NC, Chue CD, Jesky M, Stringer S, et al. (2015) Caveolin-1 single-nucleotide polymorphism and arterial stiffness in non-dialysis chronic kidney disease. Nephrol Dial Transplant. Link: https://tinyurl.com/ycg66a8p
- Tang L, Wang H, Ziolo MT (2014) Targeting NOS as a therapeutic approach for heart failure. Pharmacol Ther. Link: https://tinyurl.com/yaxkjcwm
- Massy ZA, Stenvinkel P, Drueke TB (2009) The role of oxidative stress in chronic kidney disease. Semin Dial 22: 405-408. Link: https://tinyurl.com/ycdrfc4x
- Hassan GS, Williams TM, Frank PG, Lisanti MP (2006) Caveolin-1-deficient aortic smooth muscle cells show cell autonomous abnormalities in proliferation, migration, and endothelin-based signal transduction. Am J Physiol Heart Circ Physiol 290: 2393-2401. Link: https://tinyurl.com/y6uljzz3
- Matsushita K, Sang Y, Ballew SH, Shlipak M, Katz R, et al. (2015) Subclinical atherosclerosis measures for cardiovascular prediction in CKD. J Am Soc Nephrol 26: 439-447. Link: https://tinyurl.com/ycjyt3lu
- Chand S, Holle JU, Hilhorst M, Simmonds MJ, Smith S, et al. (2013) Caveolin-1 single nucleotide polymorphism in antineutrophil cytoplasmic antibody associated vasculitis. PLoS One 8: 69022. Link: https://tinyurl.com/y8rfzqxl
- Moore J, McKnight AJ, Dohler B, Simmonds MJ, Courtney AE, et al. (2012) Donor ABCB1 variant associates with increased risk for kidney allograft failure. J Am Soc Nephrol 23: 1891-1899. Link: https://tinyurl.com/yaylsyj3
- Van der Hauwaert C, Savary G, Pincon C, Gnemmi V, Noel C, et al. (2015) Donor caveolin 1 (CAV1) genetic polymorphism influences graft function after renal transplantation. Fibrogenesis Tissue Repair 8: 8. Link: https://tinyurl.com/yaylsyj3
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
