International Journal of Radiology and Radiation Oncology
Internal mammary node positivity and autologous mastectomy reconstruction: Implications for breast cancer treatment and aesthetic outcome
Brian P Dickinson1-4*, Nikkie Vu-Huynh BS1, Monica B Vu BS1, Gregory Senofsky2,5, Judy Pham RN1, Ayushi Patel BS1, Dennis R Holmes4, Kelsey Shay3,7, Rena Callahan5 and Peter Ashjian2-4
2Providence Tarzana Medical Center, Tarzana, CA, USA
3Hoag Hospital Presbyterian, Newport Beach, CA, USA
4Glendale Adventist Medical Center, Glendale, CA, USA
5UCLA Medical Center, Hematology Oncology, Los Angeles, California, USA
6M.D, Inc., Glendale, CA, USA
7University of Southern California, Keck School of Medicine. Los Angeles, CA, USA
Cite this as
Dickinson BP, Nikkie Vu-Huynh BS, Monica B Vu BS, Senofsky G, Judy Pham RN, et al. (2021) Internal mammary node positivity and autologous mastectomy reconstruction: Implications for breast cancer treatment and aesthetic outcome. Int J Radiol Radiat Oncol 7(1): 014-021. DOI: 10.17352/ijrro.000045Objective: To discuss how the results of internal mammary lymph node biopsy at the time of autologous flap reconstruction in both the immediate and delayed setting may impact breast cancer treatment and the aesthetic outcome of autologous mastectomy reconstruction.
Background: The internal mammary vessels are primary recipient vessels for autologous breast reconstruction with deep inferior epigastric artery perforator flaps. During exposure and preparation of recipient vessels, the internal mammary lymph nodes when discovered are submitted to pathology. We have found in some patients, these internal mammary lymph nodes return positive results in patients with clinically and histologically negative axillary nodes and negative preoperative MRI/PET scan imaging. We wished to examine if these results had an impact on the radiation or chemotherapy management of the patients post-operatively. We have also sought to provide long-term follow-up on patients who have been found to have positive internal mammary lymph nodes.
Methods: We performed a retrospective review of patients with breast cancer who underwent autologous breast reconstruction with deep inferior epigastric artery perforator flaps. A specific chart review was performed on all patients found to have a positive result at the time of internal mammary lymph node biopsy.
Results: Between 2008 and 2020 a total of 18 patients with positive internal mammary lymph nodes were identified after internal mammary recipient harvest and visible lymph node biopsy. In three cases the internal mammary lymph node was positive when the axilla was negative. In 3/18 (16%) cases the patient’s stage was changed based on the incidental findings of the internal mammary nodes. Positive results changed post-operative radiation management in all patients. In only 1/16 (6%) cases was there suspicion on preoperative MRI. There were no instances of pneumothorax or other serious complications associated with the internal mammary lymph node biopsy.
Conclusions: Incidental internal mammary lymph node biopsy performed during microvascular autologous breast reconstruction may prove positive for metastatic spread despite negative pathology results of the axillary lymph nodes. This may be seen even in patients with a normal pre-op MRI. Internal mammary node biopsy is feasible without serious adverse events when performed at the time of recipient vessel dissection. If preoperative imaging or intraoperative direct examination of the internal mammary nodes raises suspicion, frozen section evaluation may change flap harvest selection to acquire more perforators and potentially alter flap inset to protect the autologous flap from the adverse effects of radiation.
Introduction
Breast cancer is common among women worldwide and is a significant cause of cancer death. Oncoplastic reconstruction of lumpectomy defects has evolved the standard of breast cancer care to eradicate cancer and optimize aesthetic outcomes [1,2]. Through the oncoplastic specialty, plastic and reconstructive surgeons have gained oncologic knowledge and become integral in the care of the breast cancer patient [2,3]. Autologous mastectomy reconstructions provide a natural consistency of the reconstructed breast with psychological benefits for the patient and are common in patients wishing to avoid implants and choosing to undergo mastectomy due to deleterious breast cancer gene mutations [4].
Lymph node status remains one of the most important factors in predicting survival in breast cancer patients [5,6]. Since the adoption of the sentinel lymph node, the status of the axillary lymph nodes has become paramount in invasive breast cancer patients to minimize morbidity and stage patients to better guide systemic therapy [7]. The axillary lymph node basin remains the predominant lymphatic drainage of the breast, while the internal mammary nodes continue to provide drainage to significant portions of the breast [8]. The status of these nodes provides additional prognostic information to potentially guide systemic therapy and/or radiotherapy [9-12]. Routine evaluation of the internal mammary lymph nodes remains cumbersome due to the relative difficulty in accessing these nodes surgically and the limitations of preoperative imaging for detection of microscopic internal mammary lymph node involvement [13]. The histologic status of the internal mammary lymph nodes carries the same prognostic significance as the status of the axillary lymph nodes and is based on the number of pathologically abnormal nodes [i.e., pN1 (1-3 positive axillary or internal mammary lymph nodes) and pN2 (4-9 positive axillary or internal mammary lymph nodes)]. When both the axillary nodes and internal mammary lymph nodes are involved, the pathological stage becomes pN3 and changes the stage to IIIC. Thus, the status of internal mammary lymph nodes may provide additional prognostic information to potentially guide systemic therapy and/or radiotherapy, as well as intraoperative plastic surgical management.
Sentinel lymph node biopsy of the internal mammary node was described in Australia where the incidence of breast cancer is higher (94.5 per 100,000) than in the United States (85.9 per 100,00) [13]. Internal mammary sentinel node biopsy has not been adopted in the United States or worldwide. During internal mammary recipient vessel dissection for autologous mastectomy reconstruction, an internal mammary lymph node is often discovered. This is often not the sentinel node; however, when we discover this node, we remove it and submit it for permanent sectioning with pathology. As we discovered positive internal mammary lymph nodes among patients with negative axillary sentinel nodes or axillary dissection, we became interested in further understanding the lymphatic drainage patterns of the breast and how they influence oncologic treatments and breast reconstruction [6]. Internal mammary lymph node positivity on PET scans could be related to breast cancer, anaplastic large cell lymphoma, or simply benign silicone lymph nodes from previous ruptured retropectoral silicone mammary prosthesis.
In the intraoperative scenario, we have been curious whether a positive frozen section of an internal mammary lymph node could change our flap harvest decision tree to include more perforators on our abdominal flap to preserve our aesthetic outcome if radiation would occur postoperatively to our flap reconstruction. Konowitz and Curtis have described this internal mammary recipient vessel site to be reliable and safe in the need of return to axillae if required by the oncologic surgeon to complete an axillary clearance [14,15]. While Hong, et. al. have described the utility of opportunistic biopsy of internal mammary lymph nodes and breast cancer treatment [16], there are no studies that describe the utility of this biopsy and the affect it may have on the reconstructive decision tree to maximize aesthetic outcome of autologous mastectomy reconstruction.
Materials and methods
A retrospective chart review was conducted on all patients who underwent autologous free flap reconstruction procedures between 2008 and 2020 by the plastic and reconstructive authors (B.D., P.A.). Among patients who underwent autologous flap reconstruction, we selected those patients who underwent flap reconstruction using the internal mammary recipient vessels. Patients who had a positive internal mammary lymph node were selected and evaluated for further chart review. The charts were then retrospectively reviewed.
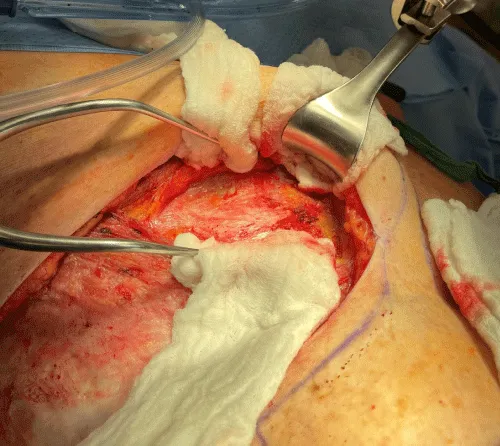
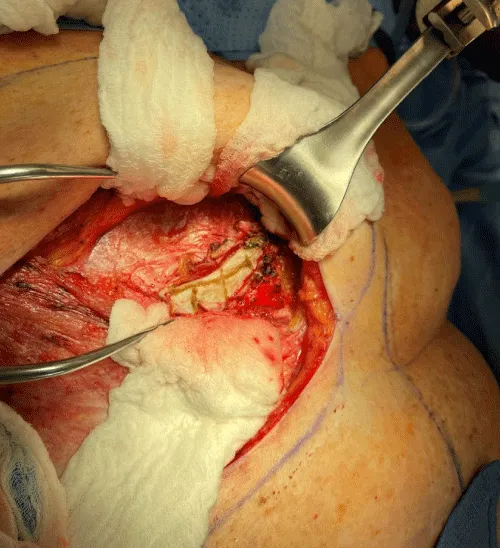
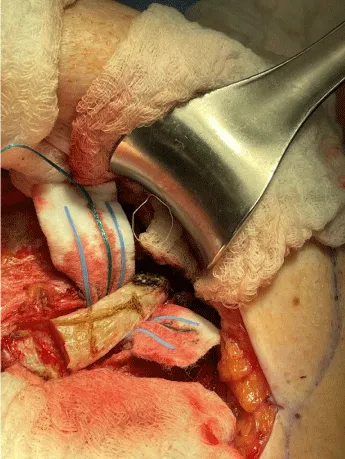
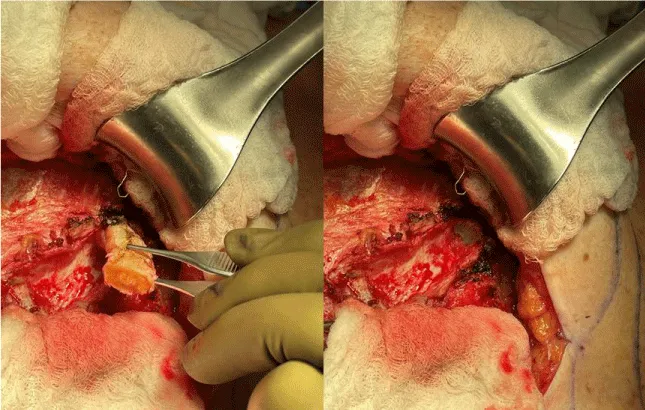
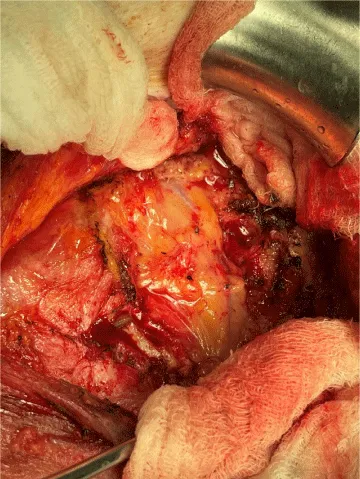
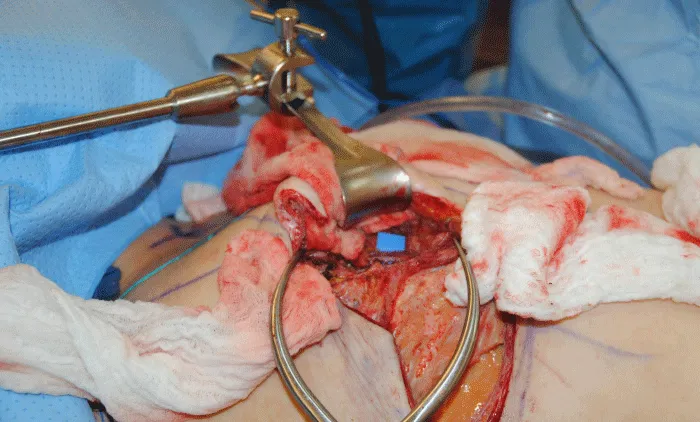
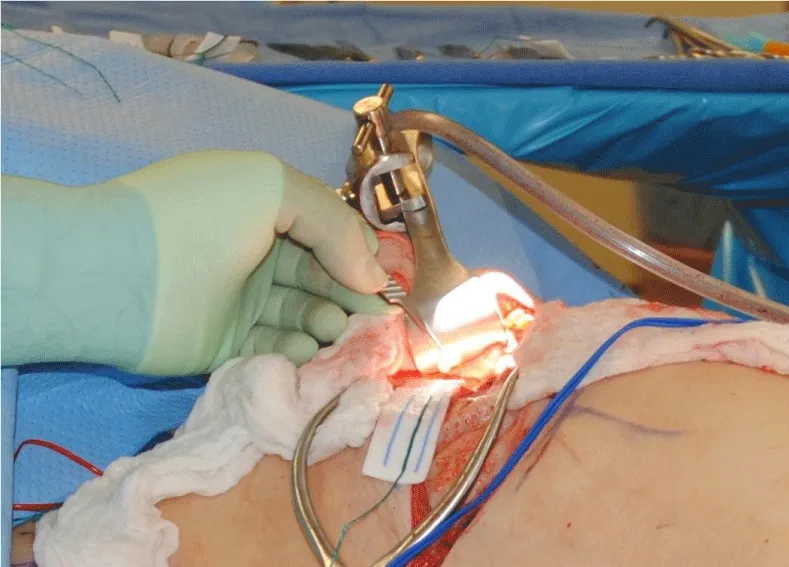
The internal mammary node harvest was done via a standard approach in all cases. The third rib was identified by counting ribs from the clavicle inferiorly to the point where the third rib could be palpated. This was done after the completion of the mastectomy in immediate cases and after the re-creation of the mastectomy defect in delayed reconstruction cases. Double hooks were placed along the direction of the pectoralis major and elevated. Bovie electrocautery was then used to dissect down to the third rib. The interspace above and below the third rib was dissected superiorly and inferiorly respectively to expose the intercostal muscles. Gelpi retractors or Goulet retractors were placed to hold the pectoralis major away from the dissection field (Figure 1). The anterior perichondrium of the third rib was scored and Freer dissection was used to elevate the anterior perichondrium off the third rib as well as the posterior perichondrium with the assistance of Doyan dissection tools (Figure 2). Once the cartilaginous section of the rib is isolated, a 1-inch x 3-inch cottonoid is used to circumferentially mobilize the posterior perichondrium (Figure 3). The segment of the cartilaginous portion of the third rib is resected (Figure 4) and the posterior perichondrium was removed as well as a portion of the intercostal muscles above and below the third rib (Figure 5). The internal mammary vessels were identified and the artery and vein were separated from each other (Figure 6). During the internal mammary vessel dissection, an internal mammary node, when identified, was dissected free and sent to pathology for microscopic examination. If the vessel diameter at the level of the third rib is insufficient for microvascular anastomosis then the dissection proceeds to the level of the second rib. If an internal mammary node is identified in this intercostal space, the internal mammary lymph node is submitted to pathology as well.
The institutional review board at the institution where the study was conducted approved the study’s ethics as chart review. Photographic consent was obtained from the patients in the chart review for presentation and publication.
Results
Between 2008 and 2020, 573 autologous mastectomy breast reconstructions were performed using the internal mammary vessels as recipient vessels1. A total of 18 patients had positive internal mammary lymph nodes after internal mammary recipient harvest and visible lymph node biopsy. Intra-operative clinical suspicion of positivity did not correlate with positivity. In three 3/18 (16%) cases the internal mammary lymph node was positive when the axilla was negative. In 3/18 (16%) cases the patient’s stage was changed based on the incidental findings of the internal mammary nodes. Positive results changed post-operative radiation management in all patients. In only 1/16 (6%) cases was there suspicion on preoperative MRI. There were no instances of pneumothorax or other serious complications associated with the internal mammary lymph node biopsy. Among the 18 positive internal mammary node patients, 16 patients underwent immediate autologous breast reconstruction and one patient underwent delayed autologous reconstruction. One patient did not receive autologous reconstruction.
Four patients had received neoadjuvant chemotherapy for inflammatory cancer and one for a positive axillary node. The locations of the tumors for the immediate reconstructions were the upper outer quadrant (n=7), central (n=8), and lower inner quadrant (n=2). The location of the tumor for the delayed reconstruction was unknown (n=1). One patient had triple-negative breast cancer and the other patients had hormone receptor-positive breast cancer.
Among the 18 patients with positive internal mammary lymph nodes, two had histologically-negative axillary sentinel nodes. Detection of positive internal mammary lymph nodes resulted in 3 patients being upstaged to a higher pathological stage. In all of the patients, the positive internal mammary lymph nodes led to additional radiation boosting to the internal mammary lymph node chain.
There were no episodes of pneumothorax, lymphatic leak, infection, or chronic seroma from the removal of internal mammary lymph nodes. There were no episodes of increased pain from the removal of internal mammary lymph nodes.
One patient who had a positive internal mammary node did not receive autologous flap reconstruction. The patient had a PET-CT with a positive internal mammary node and MRI who was staged for internal mammary node biopsy and frozen section determined positivity. Further dissection revealed involvement in the sternum and ribs. The patient did not proceed with autologous flap reconstruction which was discussed pre-operatively.
Three of the patients were deceased at the time of follow-up of the chart review. Four patients were alive with metastatic disease at the time of follow-up chart review, with many currently under control with Xeloda immunotherapy and/or tamoxifen hormone therapy. Three of the patients were known to be disease-free at the time of the study’s chart review.
Discussion
The axillary lymph node basin is the predominant lymphatic drainage of the breast. The internal mammary nodes continue to provide drainage to significant portions of the breast and may be more significant in the more elusive cancers that are non-palpable, medially located, and deep in the breast [8]. Currently, for autologous microvascular breast reconstruction, the internal mammary vessels are preferentially utilized as the recipient vessels. This puts the reconstructive microsurgeon in a position to provide the breast cancer treatment team with additional information regarding the status of these nodes without additional morbidity and time in the operating room. Despite pneumothorax as a well-described complication of these biopsies previously, we have had no episodes of pneumothorax or lymphatic leak associated with these biopsies performed concurrently with the free DIEP flap reconstruction [13].
The majority of women who require mastectomy elect to have breast reconstruction either in the immediate or delayed setting. An essential factor in both the patient’s and the plastic surgeon’s decision-making remains the requirement of Post-Mastectomy Radiotherapy (PMRT). Historically, patients with primary tumors greater than 5cm, 4 or more positive axillary lymph nodes, and positive post-mastectomy margins (ASCO), would require post-mastectomy radiotherapy to the chest wall [5]. In a more recent update to these recommendations, a joint panel of the American Society of Clinical Oncology, American Society for Radiation Oncology, and the Society of Surgical Oncology recommended unanimously that PMRT for patients with T1-2 breast cancer with 1–3 positive axillary nodes. Despite the evidence which shows a reduction of Locoregional Failure (LRF), any recurrence, and breast cancer-related mortality, they agreed that each patient’s specific situation must be addressed and these decisions modified accordingly to balance the absolute benefits of PMRT to its potential toxicities [5,17]. A recent study shows that routine chest wall PMRT does not therapeutically treat the internal mammary lymph nodes [18]. Given the general acceptance of radiation oncologists with these more liberal indications for post-mastectomy radiation.
There is no clear consensus as to the addition of radiation to the supraclavicular and/or internal mammary lymph nodes [19,20]. In the classic paper by the Danish Breast Cancer Cooperative Group 82b Trial, the addition of post-mastectomy radiotherapy to high-risk premenopausal women in addition to adjuvant chemotherapy, significantly improved locoregional recurrences and prolonged survival [21]. The radiation protocol in this study included the supraclavicular, infraclavicular, axillary, and internal mammary lymph nodes.
In 2008 Veronesi, et al. advocated for the importance of “regional nodal mapping’’ to properly stage breast carcinoma. In this study, approximately 663 patients with mostly inner quadrant breast cancers underwent biopsy of internal mammary lymph nodes [22]. They found 68 out of 663 cases (10.3%) with positive internal mammary lymph nodes. Patients with internal mammary metastases treated with radiotherapy and appropriate systemic therapy showed excellent survival (95% at 5 years). Despite the recommendation by Veronesi for exploration of the internal mammary nodes as part of the staging process for carcinomas of the medial part of the breast, it has not been universally performed [22]. A study by Poortmans, et al. showed that the addition of the regional nodes (medial supraclavicular and internal mammary lymph nodes) to early-stage breast cancer improved disease-free survival and distant disease-free survival with a reduction in breast cancer mortality [23]. The routine radiation of the internal mammary nodes is not without complications. Choi et. al. showed increased radiation pneumonitis with internal mammary node irradiation [24]. Also, Chargari et. al. showed that the addition of internal mammary lymph node irradiation contributed to the heart dose in both right- and left-sided breast cancers [25]. A study by Nilsson, et al. raised concern for the potential increased risk of stroke with radiation to the supraclavicular and internal mammary lymph nodes in breast cancer [26]. In a review article published by Freedman, et al. it was recommended that irradiation to the internal mammary lymph node chain in conjunction with the chest wall and supraclavicular reg on should be considered only for those with pathologically proven cancer involvement of the IMN [27].
Our approach to post-mastectomy breast reconstruction remains the restoration of a naturally appearing and feeling breast while minimizing complications, multiple procedures, and/or delays in any potential systemic therapy. To achieve this goal, the need for any potential post-mastectomy radiotherapy must be acknowledged and the potential operative plan adjusted to ensure the best possible outcome. The focus on the Multidisciplinary Team Approach to breast cancer treatment has allowed for these discussions to be made in a comprehensive fashion preoperatively to enable the delivery of appropriate oncological treatment in the setting of quality breast restoration. Despite studies showing the success of immediate autologous breast reconstruction in patients requiring post-mastectomy radiation, it would be preferable not to have the flap radiated. Rochlin et. al. showed that postmastectomy radiation therapy and immediate autologous breast reconstruction showed an increased probability of fat necrosis in the irradiated breast among three studies with non-irradiated controls [28]. Specific to internal mammary node radiation and autologous free flap reconstruction, Kaidar-Person, et al. showed that patients who received chest wall plus internal mammary node radiation were at significantly higher risk of fat necrosis vs patients who received only chest wall radiation [29].
Given these potential outcomes, it becomes essential to attempt to identify patients who may require radiotherapy to alter the reconstructive plan. In an attempt to prevent the flap from negatively being impacted by radiotherapy, a delay in flap surgery is often performed (referred to as the Delayed-Immediate breast reconstruction). Despite minimizing the direct effects of the radiation on the flap, it does present another problem if the internal mammary vessels have been previously radiated. Shechter, et al. showed a higher trend for flap loss (8.3% vs 0%) and vascular anastomosis failure (5.6% vs 0%) in patients with IMN radiation vs chest wall radiation alone [30]. Therefore, given the above-mentioned concerns regarding internal mammary node radiation, it is imperative to help identify those patients who may require the addition of the regional nodes n the post-mastectomy radiation field. While some patients do experience deleterious flap changes post-radiation, we have noted in some patients that radiation of an autologous flap reconstruction can be helpful to the aesthetic appearance. For example, in larger, ptotic reconstructions the radiation can help contract the skin and successfully contour the flap. In that clinical scenario patients like the non-ptotic radiated side and as plastic surgeons we perform mastopexy on the non-radiated breast to match the radiated side to make the patient happy. That being said, in those cases where a flap is necessary to close a wound and we know radiation will be employed, we intentionally keep the flap larger and harvest more venous perforators being aware of the potential radiation changes that may occur.
This paper presents our clinical experience with 18 cases where the internal mammary lymph nodes were positive for metastatic disease. The essential question remains to help determine in which patients the addition of internal mammary nodal radiation would provide a benefit while minimizing complications. Studies have shown that the inclusion of IM Node radiation in the setting of positive internal mammary lymph nodes leads to better outcomes. Sachdev, et al. showed that patients with ‘radiologic’ evidence of pretreatment abnormal internal mammary nodes were associated with advanced stage, high grade, and negative estrogen receptor status. In their population of patients, radiation to these nodes improved locoregional control [31]. These were non-biopsy proved lymph nodes. It is fair to say that nodes that become evident on preoperative imaging are more advanced. We feel that this population of patients is not what our current study includes. Without biopsy-proven abnormal internal mammary lymph nodes detected on imaging, there may be patients being assumed to have metastatic disease on pre-op imaging when in fact this is a false-positive. Mack, et al. showed that using screening MRI in high-risk patients revealed a significant percentage of women (50%) with visualized internal mammary lymph nodes (size range from 2-9mm). Patients with a new or previous diagnosis of breast cancer, prior non-breast malig ancy affecting the thorax and mediastinum, or previous radiation to the thorax were excluded from the study [32].
Some have tried to characterize preoperative MRI imaging features of metastatic internal mammary lymph nodes and correlate this with pathology and/or positron emission tomography [33]. In our experience, this is not the case. To further elucidate the impact of pathologic diagnosis of internal mammary lymph node metastasis in clinical N2b and N3b breast cancer patients. For those patients with clinically suspicious IM nodes, a fine-needle aspiration biopsy (FNAB) was attempted to confirm the diagnosis. The success rate for FNAB was 57% (40 out of 70 patients). Patients with FNAB confirmed metastatic internal mammary lymph nodes showed worse treatment outcomes vs those who had clinically diagnosed internal mammary nodes only. This is potentially because some of those patients where the imaging inferred metastatic involvement did not actually have metastatic disease [34]. Coupling the studies of Mack, et al. and Joo, et al. we can infer that the determination of the pathological involvement of internal mammary nodes in preop imaging alone is a difficult endeavor and may have a false-positive rate.
In our experience, the majority of patients with positive internal mammary nodes had a negative MRI or CT-PET. In two of the 18 patients, there was a positive MRI and PET-CT scan and these patients had advanced disease. It is helpful to know how to use the information yielded in a positive internal mammary node for oncologic treatment and flap reconstruction. From an oncologic perspective, all patients with the positive internal mammary nodes on biopsy received additional boost treatment of radiation to the internal mammary node system. Thus, our interrogation of the internal mammary node chain affected treatment 100% of the time there was a positive result when other imaging studies would not have affected the radiation management at an “early” advanced stage.
Over the last 10-12 years, there has been an evolution in the oncological treatment of the breast cancer patient. With this evolution, so too has our algorithm for breast reconstruction. Currently, the application of postoperative radiotherapy n the post-mastectomy patient has expanded. We now routinely see the recommendation of radiotherapy for patients with only 1-3 positive axillary lymph nodes and in patients who had a complete response to neoadjuvant chemotherapy based on the clinical picture at first presentation. Therefore, we currently employ a “delayed-immediate” approach to the patient who is interested in autologous reconstruction but meets the criteria for post-mastectomy radiotherapy. The goal of delayed-immediate reconstruction is to protect the autologous reconstruction from being negatively impacted by radiotherapy. A breast tissue expander along with acellular dermal matrix is used either in the pre-pectoral or retropectoral position at the time of the mastectomy in a patient with invasive breast carcinoma. This is also often employed in the patient who has had neoadjuvant chemotherapy with large tumors or positive nodes. With this “delayed immediate” approach, we are often not exploring the internal mammary lymph nodes for biopsy in the immediate setting as the expander is placed at the time of mastectomy with the internal mammary vessels and lymph node not being explored until after radiation when the expander is removed and the flap completed.
Immediate DIEP flap reconstruction is performed in prophylactic genetically-susceptible patients and in situations where a large skin paddle is required for adequate closure. For immediate DIEP reconstruction, we tend to select those patients with smaller invasive cancers and clinically negative axillary node basins to avoid the adverse effects of radiation postoperatively. Our attention is heightened during reconstruction now, based on our understanding of literature and lymph node drainage to identify the internal mammary lymph nodes intra-operatively in certain patients. During reconstruction on patients with 1) positive axillary sentinel nodes identified at mastectomy frozen section, 2) medially located tumors (lower inner quadrant), 3) non-palpable or deep tumors, and advanced tumor size we are inclined to carefully find the internal mammary node and send to pathology. In these settings, the internal mammary lymph node assessed at the time of the autologous reconstruction may affect the direction of the radiation beam. Frozen section positivity of these nodes may then affect our decision tree to harvest a “heartier” flap to revent fat necrosis and help our aesthet c result. This may also affect chemotherapy if, after neoadjuvant chemotherapy, if these nodes show evidence of residual disease, the patient may be offered an additional chemotherapy regimen. As stated earlier, we have seen instances when radiation of the autologous reconstruction can negatively affect the reconstruction (e.g., cause contraction), positively affect the reconstruction (e.g., cause tightening of a ptotic or large flap), or have minimal to no change on the reconstruction. Predicting how the patient’s reconstructed breast will react is unpredictable, but it is helpful to counsel the patient that there may be a change.
Since embarking on our breast reconstruction careers the plastic surgeon authors (BD, PA) have learned a great deal from oncologic breast surgeons, radiologists, radiation oncologists, and medical oncologists. Occasionally, when MRI and PET scans are negative and axillary sentinel lymph node biopsies are negative, we encounter a positive internal mammary lymph node that would then dictate radiation therapy or chemotherapy post-operatively and possibly alter the aesthetics of our flap reconstruction. Some would argue that the aesthetic result of a radiated flap is better than a delayed flap reconstruction following tissue expander placement. We have encountered both excellent and mediocre results in both reconstructive pathways in our experience and the literature [28-30]. As we studied the lymphatic drainage of the breast we learned that deep-seated tumors and tumors in the lower inner quadrant were more likely to have positive internal mammary lymph nodes. Deep-seated tumors are often non-palpable drain via the retromammary space to the internal mammary lymph nodes. We have become more aware of the tumor characteristic and its location in an attempt to maximize the ultimate aesthetic outcome for the breast cancer patient.
However, the autologous breast reconstruction offers the opportunity for the plastic surgeon to sample the internal mammary lymph node when exposing the recipient vessels for reconstruction. This is possible when a clinically suspicious internal mammary lymph node is discovered on MRI pre-operatively. Recipient vessel dissect on can also provide access for the oncological breast surgeon if lymphoscintigraphy shows predominant drainage to the internal mammary lymph node chain. Given our experience and research of the topic, we have a higher suspicion for deep-seated tumors regardless of quadrant and tumors in the lower inner quadrant. In these patients, we consider the use of frozen sections for internal mammary lymph nodes removed during recipient vessel exposure. A negative lymph node does not imply that the lymph nodes superior and inferior to the third intercostal space are not positive. We do not at this point advocate removing all of the lymph nodes in the chain as this could inadvertently damage recipient vessels. However, a positive lymph node on frozen section might change our flap harvest decision tree to include more perforators in our flap or muscle-sparing free TRAM to prevent the negative contractile effects of radiation on the skin post immediate flap reconstruction. This scenario is a possibility as often the recipient vessel exposure and abdominal flap harvests are occurring simultaneously.
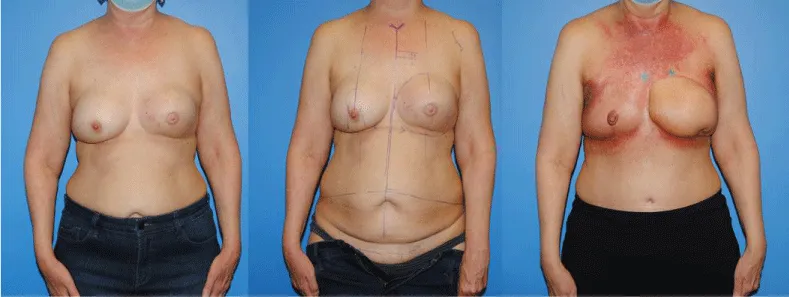
Frequently, the Deep Inferior Epigastric Artery Perforator (DIEP) flap is the primary choice for breast reconstruction. DIEP and free TRAM flaps are the most common autogenous breast reconstructive techniques used worldwide [6-8]. We inherently believe that muscle-sparing free TRAMS and or free TRAMs with excellent venous outflow have fewer radiation changes than DIEP flaps. The DIEP is based on the musculocutaneous perforators of the DIEA which course through the rectus abdominis muscle where free TRAM flaps include more muscle and more perforating vessels. Whether the radiation aesthetic differences are related to blood supply or obliteration of dead space and seroma formation, larger flaps and more bulk such as a muscle-sparing free TRAM may be better tolerated if the patient will be receiving radiation postoperatively. That is, more bulk and better blood supply may create a more radio-resistant flap. We tend to see this frequently in patients who are undergoing mastectomy for an inflammatory cancer or who have undergone neoadjuvant therapy for an inflammatory cancer and who will require radiation post-operatively (Figure 8). In these patients we would intentionally harvest a flap containing multiple arterial and venous perforators and when available, would perform a secondary anastamosis of a vein to the cephalic vein. This more robust arterial inflow and venous outflow tended to make the flap more resistant to the negative effects of radiation therapy. In many patients, the robust autologous flap did better than the surrounding native tissue of the chest wall (Figure 8).
Summary
It has been shown that standard post-mastectomy radiotherapy does not routinely include the internal mammary lymph node chain. The role of including the internal mammary lymphatic chain routinely has been controversial because there have been some questions as to its benefit and potential cardiotoxicity. However, the clinical significance of the status of the internal mammary lymph nodes has proven to be valuable to prognosis and potential guidance of systemic therapy. The reconstructive microsurgeon has a unique role in helping stratify patients based on evaluation of these nodes during 1autologous free flap reconstruction. We and others have shown these nodes can be assessed with minimal morbidity and without adding significant time to the operative procedure. The result of frozen sections of internal mammary nodes may be a factor in abdominal flap harvest and affect the aesthetic outcome of autologous breast reconstructions. Patients with internal mammary node positivity represent a population who has a significant breast cancer disease burden. The internal mammary result positivity should be taken seriously and patients should be counseled as such by all members of the breast cancer treatment team including oncologists, radiation oncologists, breast surgeons, and plastic and reconstructive surgeons. Given current immunotherapy and the possibility of immunotherapy in the future, there are opportunities for more effective counseling of these patients with significant breast cancer disease burden.
- Roland JH, Holland JC, Chaglassian T, Kinne D (1993) Psychological response to breast reconstruction: expectations for and impact on postmastectomy functioning. Psychosomatics 34: 241-243. Link: http://bit.ly/3r9H7uU
- Silverstein MJ, Mai T, Savalia N, Vaince F, Guerra L (2014) Oncoplastic breast conservation surgery: the new paradigm. J Surg Oncol 110: 82-89. Link: http://bit.ly/2OOiHKG
- Dickinson BP, Holmes D, Vu-HuynhN, Vu MB, Snyder L, et al. (2020) Autologous mastectomy reconstruction: Communication among the breast surgery team to maximize aesthetic and oncologic outcome. Breast J 26: 1771-17780. Link: http://bit.ly/3vOYi8D
- Frost MH, Schaid DJ, Sellers TA, Slezak JM, Arnold et al. (2000) Long-term satisfaction and psychological and social function following bilateral prophylactic mastectomy. JAMA 284: 319-324. Link: http://bit.ly/315qs1m
- Recht A (2016) ASCO Special Articles. 6.
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Breast Cancer (Version 3.2020).
- Zahoor S, Haji A, Battoo A, Qurieshi M, Mir W, et al. (2017) Sentinel Lymph Node Biopsy in Breast Cancer: A Clinical Review and Update. J Breast Cancer 20: 217‐227. Link: http://bit.ly/2P9OtBv
- Estourgie SH, Nieweg OE, Valdes Olmos RA, Rutger EJ, Kroon BBR (2004) Lymphatic Drainage Patterns From the Breast. Ann Surg 239: 232-237. Link: http://bit.ly/3sdbwKc
- Wright EJ, Momeni A, Kraneburg UM, et al. (2016) Clinical Significance of Internal Mammary Lymph Node Biopsy during Microsurgical Breast Reconstruction: Review of 264 Cases. Plast Reconstr Surg 137: 917e‐922e. Link: http://bit.ly/3cPfEty
- Schaverien MV, Purdie CA, Munnoch DA (2013) Clinical value of internal mammary lymph node metastases found incidentally during free flap recipient vessel exposure. Eur J Surg Oncol 39: 608‐612. Link: http://bit.ly/3dafOvV
- Yu JT, Provenzano E, Forouhi P, Malata CM (2011) An evaluation of incidental metastases to internal mammary lymph nodes detected during microvascular abdominal free flap breast reconstruction. J Plast Reconstr Aesthet Surg 64: 716‐721. Link: http://bit.ly/3c8oZ0v
- Rose JF, Zavlin D, Menn ZK, Eldor L, Chegireddy V, et. al. (2018) Implications of Internal Mammary Lymph Node Sampling During Microsurgical Breast Reconstruction. Ann Surg Oncol 25: 3134-3140. Link: http://bit.ly/3vNVCbG
- Tan C, Cargata R, Bennett I (2017) Is Sentinel Node Biopsy of the Internal Mammary Lymph Nodes Relevant in the Management of Breast Cancer? Breast J 23: 410-414. Link: http://bit.ly/391mc76
- Curtis MS, Arslanian B, Colakoglu S, Tobias AM, Lee BT (2011) Immediate microsurgical breast reconstruction and simultaneous sentinel lymph node dissection: issues with node positivity and recipient vessel selection. J Reconstr Microsurg 27: 445-448. Link: http://bit.ly/2QonX81
- Kronowitz SJ, Kuerer HM, Hunt KK, Ross MI, Massey PR, et al. (2006) Impact of sentinel lymph node biopsy on the evolution of breast reconstruction. Plast Reconstr Surg 118: 1089-1099. Link: http://bit.ly/3f0Twz9
- Hong KY, Lee HB, Yim S, Lee J, Kim TY, et al. (2017) Opportunistic Biopsy of Internal Mammary Lymph Nodes During Immediate Breast Reconstruction After Mastectomy for Breast Malignancies. Ann Surg Oncol 24: 1881-1888. Link: http://bit.ly/3c6aslW
- Fowble B, Jairam AK, Wang F, Peled A, Alvarado M, et al. (2018) Indications for Postmastectomy Radiation After Neoadjuvant Chemotherapy in ypN0 and ypN1-3 Axillary Node-Positive Women. Clin Breast Cancer 18: e107‐e113. Link: http://bit.ly/3r4bHGB
- Arora D, Frakes J, Scott J, Opp D, Johnson C, et al. (2015) Incidental radiation to uninvolved internal mammary lymph nodes in breast cancer. Breast Cancer Res Treat 151: 365‐372. Link: http://bit.ly/3cPg9DW
- Verma V, Vicini F, Tendulkar RD, Khan AJ, Wobb J, et al. (2016) Role of Internal Mammary Node Radiation as a Part of Modern Breast Cancer Radiation Therapy: A Systematic Review. Int J Radiat Oncol Biol Phys 95: 617‐631. Link: http://bit.ly/3sbkW91
- Thorsen LB, Offersen BV, Danø H, Berg M, Jensen I, et al. (2016) DBCG-IMN: A Population-Based Cohort Study on the Effect of Internal Mammary Node Irradiation in Early Node-Positive Breast Cancer. J Clin Oncol 34: 314‐320. Link: http://bit.ly/3tM3uIz
- Overgaard M, Hansen PS, Overgaard J, Hansen PS, Rose C, et al. (1997) Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 337: 949‐955. Link: http://bit.ly/3rbRqyG
- Veronesi U, Arnone P, Veronesi P, Galimberti V, Luini A, et al. (2008) The value of radiotherapy on metastatic internal mammary nodes in breast cancer. Results on a large series. Ann Oncol 19: 1553‐1560. Link: http://bit.ly/3cYWMIu
- Poortmans PM, Collette S, Kirkove C, Peignaux-Casasnovas K, Budach V, et al. (2015) Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N Engl J Med 373: 317‐327. Link: https://bit.ly/3c4BVV4
- Choi J, Kim YB, Shin KH, Ahn SJ, Lee HS, et al. (2016) Radiation Pneumonitis in Association with Internal Mammary Node Irradiation in Breast Cancer Patients: An Ancillary Result from the KROG 08-06 Study. J Breast Cancer 19: 275‐282. Link: http://bit.ly/38YTTX9
- Chargari C, Castadot P, Macdermed D, Vandekerkhove C, Bourgois N, et al. (2010) Internal mammary lymph node irradiation contributes to heart dose in breast cancer. Med Dosim 35: 163‐168. Link: http://bit.ly/311trYy
- Nilsson G, Holmberg L, Garmo H, Terent A, Blomqvist C (2009) Radiation to supraclavicular and internal mammary lymph nodes in breast cancer increases the risk of stroke. Br J Cancer 100: 811‐816. Link: http://bit.ly/2OUfpFE
- Freedman GM, Fowble BL, Nicolaou N, Sigurdson ER, Torosian MH, et al. (2000) Should internal mammary lymph nodes in breast cancer be a target for the radiation oncologist?. Int J Radiat Oncol Biol Phys 46: 805‐814. Link: http://bit.ly/3cYXENg
- Rochlin DH, Jeong AR, Goldberg L, Harris T, Mohan K, et al. (2015) Postmastectomy radiation therapy and immediate autologous breast reconstruction: integrating perspectives from surgical oncology, radiation oncology, and plastic and reconstructive surgery. J Surg Oncol 111: 251‐257. Link: http://bit.ly/2Pd533p
- Kaidar-Person O, Eblan MJ, Caster JM, Shah AR, Fried D, et al. (2019) Effect of internal mammary vessels radiation dose on outcomes of free flap breast reconstruction. Breast J 25: 286‐289. Link: http://bit.ly/3r8XZSK
- Shechter S, Arad E, Inbal A, Friedman O, Gur E, Barnea Y (2018) DIEP Flap Breast Reconstruction Complication Rate in Previously Irradiated Internal Mammary Nodes. J Reconstr Microsurg 34: 399‐403. Link: http://bit.ly/3sbnaoT
- Sachdev S, Goodman CR, Neuschler E, Kalakota K, Cutright D, et al. (2017) Radiotherapy of MRI-detected involved internal mammary lymph nodes in breast cancer. Radiat Oncol 12: 199. Link: http://bit.ly/3lOlLTb
- Mack M, Chetlen A, Liao J (2015) Incidental Internal Mammary Lymph Nodes Visualized on Screening Breast MRI. AJR Am J Roentgenol 205: 209‐214. Link: http://bit.ly/3lOlIXv
- Lee JS, Oh M, Korean Breast Cancer Society; Korean Breast Cancer Society (2014) Reproductive factors and subtypes of breast cancer defined by estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2: a register-based study from Korea. Clin Breast Cancer 14: 426‐434 . Link: http://bit.ly/2Qhxgqf
- Joo JH, Kim SS, Ahn SD, Choi EK, Jung JH, et al. (2017) Impact of pathologic diagnosis of internal mammary lymph node metastasis in clinical N2b and N3b breast cancer patients. Breast Cancer Res Treat 166: 511‐518. Link: http://bit.ly/3rdtT0d
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
