International Journal of Radiology and Radiation Oncology
Percutaneous Spine Biopsy: A Literature Review
Ali Nourbakhsh*
Cite this as
Nourbakhsh A (2015) Percutaneous Spine Biopsy: A Literature Review. Int J Radiol Radiat Oncol 1(1): 023-028. DOI: 10.17352/ijrro.000007Percutaneous spine biopsy has widely replaced open biopsy during the last 50 years. Closed biopsy is more cost effective, less invasive, and has fewer complications than open procedures. A literature search was conducted in PubMed for percutaneous spine biopsy. The contributing factors to the success of the biopsy were identified by reviewing the articles and their references. These factors included location and type of lesion, needle system and use of different modalities of imaging as well as the physician’s expertise. Potential complications include pneumothorax, hematoma, nerve root injury, transient paresis, transient spinal anesthesia, meningitis, radiculopathy, and paraplegia. Overviews of the pre and post biopsy workup along with comprehensive description of the determinants of the biopsy outcome are discussed. These determinants are: lesion type; location of the lesion in the vertebrae and the level of the spine where the lesion is located; the imaging guidance used to identify the lesion during biopsy; and, the needle or trephine which is used to obtain the specimen. A detailed review of different approaches used to penetrate the lesion by trephine or needle are also provided. The decision on how the surgeon should obtain a percutaneous spine biopsy is dependent on the characteristics of the lesion, availability of imaging guidance and also the expertise of the physician.
Introduction
Spine lesions are frequently secondary to disease processes elsewhere in the body [1]. Metastases, infections and primary tumors are the most common lesions of the vertebrae. Adequate treatment of these lesions depends on the accurate histological diagnosis [2]. Although clinical features and laboratory and imaging studies are important contributors to diagnosis, biopsy is often needed to confirm the diagnosis and guide treatment [3]. Open biopsy is considered to be the so-called gold standard for diagnosis of bone lesions, with 98% accuracy [4]. Shortcomings associated with open biopsy include skin, bone, and soft tissue problems (up to 17%), the risk of a diagnosis error (up to 18%), and the risk of missing a small lesion [5]. Percutaneous biopsy is becoming increasingly more common. However the accuracy can be variable depending on the location and type of the lesion, approach and type of needle and imaging used for obtaining the specimen.
Closed biopsy is more cost effective, less invasive, and has fewer complications than open biopsy [4]. Additionally, monitoring of nerve root function during the procedure minimizes the morbidity if it is done under local anesthesia [6]. However, the accuracy of percutaneous biopsy is lower than open biopsies, and a second biopsy may be required for definitive diagnosis [1].
Percutaneous bone biopsy was first described by Coley and Martin in the early 1930s and used for spine lesions in 1935 by Robertson and Ball [8-10]. Trephine was first used by Siffert et al. in 1949, for the biopsy of a sclerotic vertebral lesion. They also introduced the use of radiographs during the procedure [11]. The first large series of spine biopsies (1061) was reported by Ottolenghi in 1955 [12]. Such early reports described various methods of blind insertion of the needle. However, the introduction of the fluoroscopy-guided biopsy made such techniques obsolete in almost a decade [13]. In 1981, Adapon described the use of CT scan for biopsy guidance, which was used 10 years later for transpedicular biopsy by Renfrew [14,15].
The important roles of the vertebral body biopsy are (a) identification of an unknown lesion before treatment can be started, (b) exclusion of metastasis in patients with a known primary tumor, (c) identification of the organism in spondylodiscitis where the other cultures (blood, urine, etc.) are negative, (d) failure of therapy, (e) intractable or increasing back pain with either vertebral compression fracture or Paget’s disease, (f) exclusion of malignancy in intractable discitis in children [16].
Review
Location and type of lesion, needle system and use if different modalities of imaging have been mentioned as determinants of accuracy the accuracy of spine biopsy. Adequacy of the sample is considered to be dependent on the accurate localization of the lesion, size of the tissue biopsy core, and lack of architectural distortion, which are determined by trephine and the nature of the lesion. Radiation prior to biopsy may impair the pathologic diagnosis [17].
The overall complication rate of spine biopsy varies from 0 to 10% [18]. Potential complications include pneumothorax, hematoma, nerve root injury, transient paresis, transient spinal anesthesia, meningitis, radiculopathy, and paraplegia [19]. Thoracic spine biopsy was discouraged due to associated complications in early studies, but the advent of sophisticated imaging provided more a precise picture of the vital structures and increased the safety of this procedure in the cervical and thoracic spine [20]. In 75 to 90% of thoracic biopsies definitive tissue can be obtained and the complication rate has been reported to be 0 to 21% [18].
Excision of the percutaneous biopsy track is mandatory when performing a second procedure [18,21].
Prebiopsy work-up
The prebiopsy work-up should include (a) routine spine radiographs and CT scan; (b) identification of vital structures that can potentially be damaged and evaluation of the hypervascularity of the lesion; and (c) complete blood count, activated partial thromboplastin time (APTT), and prothrombin time (PT) [1,4,14]. In addition, aspirin and nonsteroidal anti-inflammatory drugs should be discontinued 7 days before the biopsy [2]. This can decrease the incidence of epidural hematoma and possible neurologic complication if the needle passes adjacent to the spinal cord or causes dural tear. Different methods of specimen handling and fixation mandate pre-procedure consultation of the physician who obtains the biopsy with the pathologist. This ensures that adequate number of specimens are obtained and proper tests are ordered which can include cultures, gram stain, cytogenetic and histology tests [22]. The only absolute contraindication of biopsy is an uncorrected bleeding tendency [17]. It should never be overlooked that the biopsy should be performed at the center where the ultimate treatment of the lesion will be done. It has also been proven that the complication rates are lower in referral centers [4].
Postbiopsy care
Monitoring the patient (varies from 1 hour to overnight observation) [14]. In cases of wide bore trephine use, it is advised to keep the patient nil by mouth (NPO) for 24 hours as a precaution for ileus [22]. A chest radiograph should be taken after thoracic spine biopsy [23]. Postoperative pain management, laxatives, hydration as well as cardiopulmonary monitoring for high risk patients or procedure are recommended.
Factors determining the outcome
Lesion type: Lytic, mixed lytic, compression fractures, and inflammatory bone lesions [24], have the highest accuracy rate (93%). Biopsy of sclerotic lesions can have just 76% accuracy. The lesions with no CT scan evidence of tumor also have low accuracy rate (81%) [25]. Biopsy of the soft bone or granulation yields more sufficient material than sclerotic bone [14]. Ghelman reported that the major causes of inaccuracy are sclerotic bone lesions [2]. However, compression fractures have been found to give both high and low accuracy rates in different studies by Kattapuram et al., and Jacobsson [20,24], Stoker et al., suggest that, in cases of sclerotic bone lesions, the needle should be directed towards the least dense area of the lesion because they are difficult to penetrate and the sample often shows fragmented spicules of bone with artifact [23]. Metastatic lesions provide the highest accuracy rate [20]. This might be due to the fact that the physician has the option of doing the procedure on the largest or most accessible lesion in situations of multiple metastasis. Similarly, the highly malignant, very cellular lesions have high rates of diagnostic yield. Giant cell tumor and eosinophilic granuloma are well diagnosed by cytological examination of the smear [57].
The histologic examination of the osseous blood increases the accuracy of the biopsy and it should be treated as a tissue specimen [26]. The advantage of fine needle aspiration biopsy (FNAB) is that the pathologist can render the preliminary diagnosis at the time of biopsy. This primary diagnosis is especially satisfactory in cases of infection and metastasis [20].
Any tissue or fluid obtained should be sent for culture [5]. In some instances the cystic or necrotic lesions may not yield any tissue in the first passage. Injection of a contrast medium into the cystic area may delineate a solid portion, which then can be biopsied. No change of cell morphology has been reported by this procedure [23].
The combination of aspiration and biopsy is preferred, especially in cases of tuberculosis (TB) where culture of the organism is difficult and delayed. In these situations, histology may reveal the presence of caseos granuloma, which confirms the diagnosis [27].
Closed biopsy, as mentioned by Fyfe, should not be done in thoracic lesions with cord compression because of further displacement of the tumor. In these cases open biopsy and decompression is the best option [28]. Hypervascularity of the lesion does not cause any problem, since the biopsy track is small enough to preserve the supporting tissue [23].
Taking several cores and removing the plugs of cortical bone from the needle before advancing it into a soft lesion can minimize the biopsy failure [29]. However obtaining more than three biopsies of the lesion does not increase the likelihood of positive results [16].
Open biopsy may be considered in cases of destroyed posterior vertebral body cortex, significant scoliosis, and severe compression fracture of the thoracic spine. Highly vascular lesions with invasion to the posterior vertebral cortex are another possible indication [30].
Spondylodiscitis
Although the accuracy of the histopathologic examination for the diagnosis of spondylodiscitis is high, microbiologic evaluation generally identifies the causative organism in only 35-60% of cases [31]. A higher micro-organism isolation rate can be expected when pus is obtained during biopsy [23]. In TB cases, more advanced stages of the disease result in nonspecific results due to the start of the healing process [1]. Likewise, in chronic spondylodiscitis the micro-organisms exist in isolated and adherent colonies, which decreases the adequacy of the specimen [32]. It has been proven that the prebiopsy antibiotic therapy is an important determinant of failure to isolate the causative organism [33]. White showed that the addition of a histology examination increases the sensitivity of the culture for the diagnosis of spondylodiscitis by almost 40%. Negative results in the presence of high clinical suspicion necessitates a repeat biopsy before institution of prolonged antibiotics therapy [32].
If anaerobics are the expected causative organisms, rapid handling to the microbiologist is necessary. When an infection is suspected and no fluid could be obtained, several sterile saline injections and aspirations should be tried. Culture of the tissue specimens is the last resort [27].
Due to the low adequacy and low rate of organism identification in spondylodiscitis, especially in cases of TB and chronic infections as well as when antibiotic therapy was given previously, the use of large bore needle and multiple passes during biopsy are necessary. The small-caliber needles generally do not allow for sampling of the adjacent vertebrae, and viscous infected fluid can be difficult to aspirate [34].
Location of the lesion
Indications for using either the posterolateral or transpedicular approach are determined by the location of the lesion. Posterolateral approach the needle is inserted through the paraspinal muscles directly into the disc space or vertebral body while the transpedicular approach uses the pedicle as the corridor to get to the vertebral body. For lesions that are inaccessible by a posterolateral approach due to needle path obstruction by a transverse process, a rib, or the iliac crest (like small postcentral lesions or even the posterior half of the vertebra), a transpedicular approach is preferred. It is also appropriate for the lesions of the pedicle [35,36]. Lesions of the intervertebral disc are accessible by the posterolateral route and through the pedicle of the lower vertebra by the transpedicular approach [31,37]. Lesions of the lower vertebral body can be approached by the posterolateral route [36]. Layton showed that using a transpedicular approach (by a modified vertebroplasty approach), permits sampling of the disc space, as well as both adjacent vertebral endplates that can be utilized for biopsy of suspected discitis/spondylodiscitis [34]. Hsu mentioned that lesions of the lateral lumbar vertebral wall are better biopsied through a posterolateral approach [35]. In case of lesions of the entire vertebral body, the transpedicular approach is preferred [38].
Needle selection
The majority of the spine lesions are metastatic lesions that have high diagnostic yield and even use of needles with low IDs can provide satisfactory specimens. Kattapuram’s study showed that there is a slight increase (not statistically significant) in the accuracy of the biopsy with large needles than fine needles [20].
In a cadaveric study, Fyfe showed that a biopsy diameter of more than 2 mm increases the diagnostic yield of the specimen from 59% to 90% [28]. In a similar study, Ward et al., concluded that samples obtained with the 3.5 mm trephine are more suitable for histology exam than 2 mm trephine [37]. Logan et al., found an increased accuracy of the samples with core biopsy than fine needle sampling [39].
FNAB helps immediate evaluation of the adequacy of the material by frozen section examination, which may minimize the number of passes and can be especially helpful in difficult locations like the cervical spine [20]. On the other hand, core biopsies help make a more accurate diagnosis [40]. If FANB is used, it is important to do a frozen section or rapid cytological exam of the lesions that are necrotic or heterogeneous on the imaging studies [41]. Although the diagnostic yield is higher with large bore needles (due to larger amount of tissue and less crush artifact, they carry the disadvantage of obscuring the image on CT images and maceration of soft tissue paraspinal mass (small gauge needles are more appropriate for these lesions) [18,37].
For soft or superficial lesions, a Tru-cut needle is more appropriate, while Jamshidi and Coombs needles are better for hard bone [5]. For sclerotic or primary bone lesions or diagnosis of metabolic disorders, large needle diameters are needed [28]. Faugere stated that for the diagnosis of quantitative evaluation of metabolic bone diseases the core size of the specimen should not be less than 3 mm [42].
In the presence of extraosseous extension of the tumor or if the needle could be passed through a thinner bony shell, even small bore needles may provide adequate specimens [41]. Large bore needle are preferable for primary bone tumors, while cytologic aspirates can provide satisfactory specimens for metastatic lesions [20].
Some of more popular needles are as follows: Craig needle (ID: 3.5 mm), Harlow Wood needle (ID: 3mm), Jamshidi needle (ID: 3.1mm), Jamshidi and Swaim needle (ID: 2mm), Ackerman needle (ID: 1.5mm), Tru-cut needle (ID: 1.8), and Laredo needle (ID: 1.6) [21,28,43-46].
Mixed lesions, or lytic lesions with an intact overlying cortex, require the use of a trephine to cut a window through the overlying cortical bone [47]. Where the use of needles with low IDs is highly effective, such as in metastatic lesions, and due to the higher complication rate associated with large bore needles, the clinician can consider a needle with a smaller ID as the first method of obtaining biopsy in such lesions. On the other hand, in primary bone lesions, lesions covered by an intact cortex, and sclerotic tumors, the use of wider bore needles is more feasible despite the higher complication rate. The patient should be informed of such complications in these situations.
Approaches
Posterior or direct approach is used for all parts of the spine through a mid-sagittal or parasagittal route while the patient is in the prone position [35]. The procedure can be done under local anesthesia except in patients who are unable to remain still, such as children or when local tenderness is present, where general anesthesia is used [17]. Moreover, with local anesthesia the physician can benefit from cooperation of the patient to avoid nerve damage or promptly manage complications like pneumothorax [48]. The entry point should be placed as far as possible from the infected or irradiated sites [29]. When performing procedure it is important to make sure that the biopsy tract can be resected in possible subsequent surgery. Involvement of the surgeon in decision making is recommended in such situations. Anterior approaches are safer in the cervical spine since the vitals structures can be damaged by an anterior approach in the thoracic spine. Such approaches should be discussed between the physician obtaining the biopsy and the surgeon to make sure that the tract can be excised along with the lesion in possible subsequent operations.
Cervical Spine:
1. Anterolateral (Figure 1): It is carried out for C2-C7 lesions, while the patient is in supine position. The needle is passed between the airway and the carotid artery while the examiner pushes the contents of the carotid sheet away from the tip of the needle. In C2-C3 lesions, the needle is directed medially and superiorly to slide below the jaw [49]. This approach was also successfully used in taking a biopsy from lateral masses of C1 [50].
2. Trans-oral: It is recommended for the lesions of the first three cervical vertebras to avoid damaging the surrounding structures. Angiography may be used to visualize the vessels accurately [49].
3. Posterolateral (Figure 1): It has been used for C4 to C7 vertebral lesions. The needle is advanced between the posterior edge of the sternocleidomastoid (SCM) muscle and the vertical line traced from the tip of mastoid. It is safer in terms of injury to the vessels and brachial plexus [49].
These procedures should be performed only if adequate surgical and neurosurgical support is available, due to the risk of airway or spinal canal compromise [50]. Transpedicular approach is rarely used for cervical spine lesions [51].
Thoracic (T) and lumbar (L) spine
Posterolateral (T&L): The patient is in the prone or decubitus position and the needle is introduced lateral to the transverse process (5-7 cm from the midline18) and the tip is aimed at the lateral side of the vertebral body. If possible, it is safer to perform the thoracic biopsies from the right side to avoid puncturing the aorta and the lumbar biopsies from the right to avoid the inferior vena cava vein [1,2]. The posterolateral approach has been used successfully in the presence of a posterior fixation device [46].
Transcostovertebral (T) (Figure 2): With the patient in the prone or lateral position the needle is advanced between the anterior aspect of transverse process and the posterior part of the rib neck and then it is directed through the costovertebral joint to the lesion. It is well tolerated because of the distance from the intercostal nerves. Also, it avoids the risk of pleural puncture and angulation of the needle in the craniocaudad direction [51].
Transpedicular (T&L) (Figure 2): The patient is placed in the prone position and the needle is introduced along the course of the pedicle to penetrate the vertebra at the groove between the lateral aspect of the superior articular facet (mamillary process) and the transverse process. In this area the cortex is typically thin and the biopsy needle is perpendicular to the bone, decreasing the incidence of slippage. More than 50% of the cancellous bone the vertebral body is accessible through this approach. The shorter and less oblique tracks of the needle provide easier en block resection of the tumor than the posterolateral approach. The potential risk of this procedure is violation of the inferior and medial wall of the pedicle, which may lead to hematoma formation, dissemination of the tumor, or infection into the spinal canal, which can give rise to spinal cord compromise. Inadvertent puncture of the aorta and vena cava due to deep penetration of the needle are other potential risks. Consideration of the external diameter of the trephine and the internal width of the pedicle is always mandatory in this approach. The needle should always be towards the lateral and superior wall of the pedicle. The needle is placed with more caudal to cranial angulation than a traditional vertebroplasty approach in an attempt to provide subsequent access to the intervertebral disk the endplate biopsy would also be feasible [15,19].
Transforminodiscal (T&L): The superior concave surface of the vertebra is the entry point of the needle. The superomedial part of the vertebra is not accessible by this approach [52].
The anterior approach also can be used when the lesion is confined to the anterior body. In such cases the distance between the lesion and the abdominal wall should not be too great or the accuracy of the biopsy will be affected [35].
Sacrum
• Sacral lesions are more accessible by surgery and the rate of metastasis to this area is lower than for other parts of the spine. Primary bone tumors like chordoma and giant cell tumor have a propensity for the sacrum as opposed to other spinal locations. As discussed in the lesion type section, these primary tumors are not accurately diagnosed by closed biopsy. It is shown that there will be a significant delay in treatment if the initial biopsy is a percutaneous biopsy rather than an open biopsy. The accuracy of open biopsy is much higher [53]. For sacral lesions, either a posterior or posterolateral approach may be used depending on the location of the lesion [54].
Imaging guidance
CT-guided biopsy provides a more accurate image of the vital structures around the lesion. The reported accuracy of CT-guided spinal bone biopsy is 67-97%, and the complication rate ranges from 0-26% [2].
Some lesions may be inaccessible under fluoroscopy guidance due to either the small size or because the anatomic site may be obscured by overlying bony structures [55]. Additionally, visualization of the neural arch and paravertebral lesions are difficult by fluoroscopy, while CT scan ensures accurate biopsy of these lesions [2]. On the other hand, real time imaging is not possible with CT scan and this will not only lengthen the time of the procedure, but also increases the risk of needle slippage into the adjacent structures while trying to penetrate the bone [18].
Since fluoroscopy is done in the operating room (OR), possible complications can be addressed immediately. Moreover, fluoroscopy is performed in aseptic conditions and the trochar can be followed while reaching the vertebra that reduces the risk of moving about and so the risk of asepsis. These are rarely fulfilled in CT room. In cases where fluoroscopy cannot identify the lesion or in paraspinal soft tissue tumors, the use of CT scan is needed. Additionally, in difficult areas, like the upper thoracic and cervical spine, the use of CT scan is much safer [5]. The use of a computed tomography scan did not increase the adequacy and accuracy of the samples significantly. The complication rate of biopsies obtained by computed tomography was 3.3%, compared with 5.3% for fluoroscopy [55].
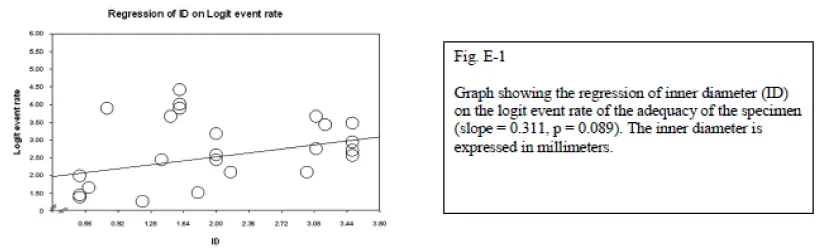
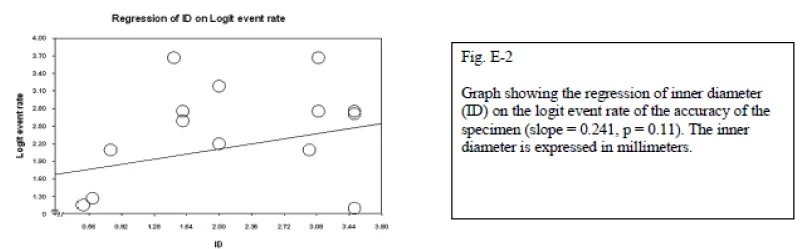
Ultrasonography-guided biopsy is used in the cervical spine and lumbar spine without any reported complications. The reasons for its consideration were cost effectiveness, real-time monitoring, and avoidance of ionizing radiation. It is not feasible for deeply located bone lesions without invasion to the cortex; however, it is successfully used for paraspinal or posterior elements lesions. Sonography has not been considered a safe method in the thoracic spine due to the presence of air in the lungs, which precludes satisfactory visualization of the lesion. The only exception to this rule is posterior element lesions [57]. Nourbakhsh et al., did a meta-analysis on different methods of percutaneous spine biopsy. They found that although the adequacy, and accuracy of biopsy increases with increased inner diameter of the needle, the complication rate also increases significantly (p = 0.01).
Conclusion
The determinants of the type of approach, instruments, and imaging used for spine biopsy include: lesion type, location of the lesion in the vertebrae, and the level of the spine where the lesion is located. A detailed review of different approaches used to penetrate the lesion, the imaging guidance used to identify the lesion during biopsy; and, the instruments, which can be used to obtain the biopsy, are provided. The decision also depends on the availability of imaging guidance and the expertise of the physician. There might be a steep learning curve to gain expertise in the wide variety of approaches used to obtain adequate specimen.
- Odendaal T, Lemmer LB (1991) The value of percutaneous trephine biopsy in the diagnosis of lesions of the vertebral column. S Afr Med J 79: 21-23.
- Ghelman B, Lospinuso MF, Levine DB, O'Leary PF, Burke SW (1991) Percutaneous computed-tomography-guided biopsy of the thoracic and lumbar spine. Spine (Phila Pa 1976) 16: 736-739.
- Tehranzadeh J, Freiberger RH, Ghelman B (1983) Closed skeletal needle biopsy: review of 120 cases. AJR Am J Roentgenol 140: 113-115.
- Altuntas AO, Slavin J, Smith PJ, Schlict SM, Powell GJ, et al. (2005) Accuracy of computed tomography guided core needle biopsy of musculoskeletal tumours. ANZ J Surg 75: 187-191.
- Babu NV, Titus VT, Chittaranjan S, Abraham G, Prem H, Korula RJ, et al. (1994) Computed tomographically guided biopsy of the spine. Spine 19: 2436-2442.
- Hadjipavlou AG, Kontakis GM, Gaitanis JN, Katonis PG, Lander P, et al. (2003) Effectiveness and pitfalls of percutaneous transpedicle biopsy of the spine. Clin Orthop Relat Res : 54-60.
- Fraser-Hill MA, Renfrew DL, Hilsenrath PE (1992) Percutaneous needle biopsy of musculoskeletal lesions. 2. Cost-effectiveness. AJR Am J Roentgenol 158: 813-818.
- Coley BL, Sharp GS, Ellis EB (1931) Diagnosis of bone tumors by aspiration. Am J Surg 13: 215-224 .
- Martin HE, Ellis EB (1930) Biopsy by needle puncture and aspiration. Ann Surg 92: 169-181.
- Robertson RC, Robert PB (1935) Destructive spine lesions. JBJS 17: 749-758.
- Siffert RS, Arkin AM (1949) Trephine biopsy of bone with special reference to the lumbar vertebral bodies. J Bone Joint Surg Am 31A: 146-149.
- Ottolenghi CE (1955) Diagnosis of orthopaedic lesions by aspiration biopsy; results of 1,061 punctures. J Bone Joint Surg Am 37-A: 443-464.
- Rabinov K, Goldman H, Rosbash H, Simon M (1967) The role of aspiration biopsy of focal lesions in lung and bone by simple needle and fluoroscopy. Am J Roentgenol Radium Ther Nucl Med 101: 932-938.
- Adapon BD, Legada BD Jr, Lim EV, Silao JV Jr, Dalmacio-Cruz A (1981) CT-guided closed biopsy of the spine. J Comput Assist Tomogr 5: 73-78.
- Renfrew DL, Whitten CG, Wiese JA, el-Khoury GY, Harris KG (1991) CT-guided percutaneous transpedicular biopsy of the spine. Radiology 180: 574-576.
- Ozsarlak O1, De Schepper AM, Wang X, De Raeve H (2003) CT-guided percutaneous needle biopsy in spine lesions. JBR-BTR 86: 294-296.
- Möller S, Kothe R, Wiesner L, Werner M, Rüther W, Delling G, et al. (2001) Fluoroscopy-guided transpedicular trocar biopsy of the spine--results, review, and technical notes. Acta Orthop Belg 67: 488-499.
- Metzger CS, Johnson DW, Donaldson WF 3rd (1993) Percutaneous biopsy in the anterior thoracic spine. Spine (Phila Pa 1976) 18: 374-378.
- Stringham DR, Hadjipavlou A, Dzioba RB, Lander P (1994) Percutaneous transpedicular biopsy of the spine. Spine (Phila Pa 1976) 19: 1985-1991.
- Kattapuram SV, Khurana JS, Rosenthal DI (1992) Percutaneous needle biopsy of the spine. Spine (Phila Pa 1976) 17: 561-564.
- DeSantos LA, Lukeman JM, Wallace S, Murray JA, Ayala AG, et al. (1978) Percutaneous needle biopsy of bone in the cancer patient. AJR Am J Roentgenol 130: 641-649.
- Alexander AH (1988) Thoracolumbar needle biopsy. Orthopedics 11: 1473-1477.
- Stoker DJ, Kissin CM (1985) Percutaneous vertebral biopsy: a review of 135 cases. Clin Radiol 36: 569-577.
- Jacobsson H (1982) Percutaneous bone biopsy with a simple punch instrument. Indications, results and complications. Acta Radiol Diagn 23: 415-422.
- Lis E, Bilsky MH, Pisinski L, Boland P, Healey JH, O'malley B, Krol G, et al. (2004) Percutaneous CT-guided biopsy of osseous lesion of the spine in patients with known or suspected malignancy. AJNR Am J Neuroradiol 25: 1583-1588.
- Hewes RC, Vigorita VJ, Freiberger RH (1983) Percutaneous bone biopsy: the importance of aspirated osseous blood. Radiology 148: 69-72.
- Murphy WA, Destouet JM, Gilula LA (1981) Percutaneous skeletal biopsy 1981: a procedure for radiologists--results, review, and recommendations. Radiology 139: 545-549.
- Fyfe IS, Henry AP, Mulholland RC (1983) Closed vertebral biopsy. J Bone Joint Surg Br 65: 140-143.
- Moore TM, Meyers MH, Patzakis MJ, Terry R, Harvey JP Jr (1979) Closed biopsy of musculoskeletal lesions. J Bone Joint Surg Am 61: 375-380.
- Jelinek JS, Kransdorf MJ, Gray R, Aboulafia AJ, Malawer MM (1996) Percutaneous transpedicular biopsy of vertebral body lesions. Spine 21: 2035-2040.
- Christodoulou A, Zidrou C, Savvidou OD, Givissis P, Apostolou T, et al. (2005) Percutaneous Harlow Wood needle biopsy of the spine: a retrospective analysis of 238 spine lesions. Orthopedics 28: 784-789 .
- White LM, Schweitzer ME, Deely DM, Gannon F (1995) Study of spondylodiscitis: utility of combined histologic and microbiologic evaluation of percutaneous biopsy samples. Radiology 197: 840-842.
- Cotty P, Fouquet B, Pleskof L, Audurier A, Cotty F, et al. (1988) Vertebral spondylodiscitis: value of percutaneous biopsy. 30 cases. J Neuroradiol 15: 13-21.
- Layton KF, Thielen KR, Wald JT (2006) A modified vertebroplasty approach for spine biopsies. AJNR Am J Neuroradiol 27: 596-597.
- Hsu WC, Lim KE (2001) Computed tomography-guided percutaneous transpedicular biopsy of the thoracic spine. Chang Gung Med J 24: 368-375.
- Phadke DM, Lucas DR, Madan S (2001) Fine-needle aspiration biopsy of vertebral and intervertebral disc lesions: specimen adequacy, diagnostic utility, and pitfalls. Arch Pathol Lab Med 125: 1463-1468.
- Ward JC, Jeanneret B, Oehlschlegel C, Magerl F (1996) The value of percutaneous transpedicular vertebral bone biopsies for histologic examination. Results of an experimental histopathologic study comparing two biopsy needles. Spine (Phila Pa 1976) 21: 2484-2490.
- Pierot L, Boulin A (1999) Percutaneous biopsy of the thoracic and lumbar spine: transpedicular approach under fluoroscopic guidance. AJNR Am J Neuroradiol 20: 23-25.
- Logan PM, Connell DG, O'Connell JX, Munk PL, Janzen DL (1996) Image-guided percutaneous biopsy of musculoskeletal tumors: an algorithm for selection of specific biopsy techniques. AJR Am J Roentgenol 166: 137-141.
- Akhtar I, Flowers R, Siddiqi A, Heard K, Baliga M (2006) Fine needle aspiration biopsy of vertebral and paravertebral lesions: retrospective study of 124 cases. Acta Cytol 50: 364-371.
- Dupuy DE, Rosenberg AE, Punyaratabandhu T, Tan MH, Mankin HJ (1998) Accuracy of CT-guided needle biopsy of musculoskeletal neoplasms. AJR Am J Roentgenol 171: 759-762.
- Faugere MC, Malluche HH (1983) Comparison of different bone-biopsy techniques for qualitative and quantitative diagnosis of metabolic bone diseases. J Bone Joint Surg Am 65: 1314-1318.
- Craig FS (1956) Vertebral-body biopsy. J Bone Joint Surg Am 38-A: 93-102.
- Jamshidi K, Swaim WR (1971) Bone marrow biopsy with unaltered architecture: a new biopsy device. J Lab Clin Med 77: 335-342.
- Ackermann W (1963) Trephine use for bone biopsy. JAMA 184: 11-14.
- Laredo JD, Bard M (1986) Thoracic spine: percutaneous trephine biopsy. Radiology 160: 485-489.
- Kornblum MB, Wesolowski DP, Fischgrund JS, Herkowitz HN (1998) Computed tomography-guided biopsy of the spine. A review of 103 patients. Spine 23: 81-85.
- Bender CE, Berquist TH, Wold LE (1986) Imaging-assisted percutaneous biopsy of the thoracic spine. Mayo Clin Proc 61: 942-950.
- Tampieri D, Weill A, Melanson D, Ethier R (1991) Percutaneous aspiration biopsy in cervical spine lytic lesions. Indications and technique. Neuroradiology 33: 43-47.
- Kattapuram SV, Rosenthal DI (1987) Percutaneous biopsy of the cervical spine using CT guidance. AJR Am J Roentgenol 149: 539-541.
- Brugières P, Gaston A, Voisin MC, Ricolfi F, Chakir N (1992) CT-guided percutaneous biopsy of the cervical spine: a series of 12 cases. Neuroradiology 34: 358-360.
- Sucu HK, Bezircioglu H, Ciçek C, Erşahin Y (2003) Computerized tomography-guided percutaneous transforaminodiscal biopsy sampling of vertebral body lesions. J Neurosurg 99: 51-55.
- Ozerdemoglu RA, Thompson RC Jr, Transfeldt EE, Cheng EY (2003) Diagnostic value of open and needle biopsies in tumors of the sacrum. Spine 28: 909-915.
- Settle WJ, Ebraheim NA, Coombs R, Saunders RC, Jackson WT (1990) CT-guided biopsy of metastatic sacral tumors. Orthopedics 13: 753-758.
- Gatenby RA, Mulhern CB Jr, Moldofsky PJ (1984) Computed tomography guided thin needle biopsy of small lytic bone lesions. Skeletal Radiol 11: 289-291.
- Nourbakhsh A, Grady JJ, Garges KJ (2008) Percutaneous spine biopsy: a meta-analysis. J Bone Joint Surg Am 90: 1722-1725.
- Gupta S, Takhtani D, Gulati M, Khandelwal N, Gupta D, et al. (1999) Sonographically guided fine-needle aspiration biopsy of lytic lesions of the spine: technique and indications. J Clin Ultrasound 27: 123-129.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
