Global Journal of Cancer Therapy
Nav channels in cancers: Non-classical roles
Hengrui Liu*
Cite this as
Liu H (2020) Nav channels in cancers: Non-classical roles. Glob J Cancer Ther 6(1): 028-032. DOI: 10.17352/2581-5407.000032Voltage-gated sodium channels (Nav channels) are transmembrane proteins that allow the permeability of sodium ions across membranes. They are critical for the initiation of the action potential. Research has revealed that Nav channel α and β subunits expressed abnormally and play non-classical roles in some cancer types. However, the understanding of Nav channel proteins in cancers remains insufficient and the lack of specific Nav channel drugs impedes the study in this field. The purpose of this mini-review is to summarize and comment on the present understanding of the roles of Nav channels in cancers. The membrane potential in non-excitable cells is critical for cell activities. Cancer cells usually have a higher resting membrane potentials than non-cancer cells. Nav1.5 and Nav1.7 are the two most expressed Nav channels in human cancers, while Nav1.2 and Nav1.6 are also expressed in some cancer types. There are several mechanistic speculations for the role of Nav channels in cancer, which include sodium-other ions homeostasis, the β subunits, and humoral regulation. To be mentioned, the review includes some personal perspectives that are required for further validation. This review will be conducive to the field of Nav channels in cancers.
Classical roles of Nav channels
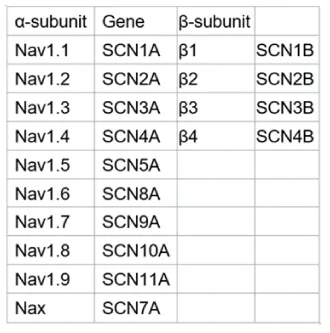
Voltage-gated sodium channels (Nav channels) are transmembrane proteins that allow the permeability of sodium ions across membranes. They have been known as critical proteins for the initiation of the action potential. The Nav channels are comprised of α subunits and β subunits. To date, a total of nine Nav channel α subunit isoforms (Nav1.1–1.9) and four types of β subunits (β1-4) have been identified in different tissues, which are encoded by “SCN_A/B“ genes (Figure 1) [1,2]. As one of the major channels participate in action potential, Nav channels play a critical role in neurons and other electrically excitable tissues. In this mini-review, I will introduce Nav1.5 and Nav 1.7 as two examples and briefly summarize the function of β subunits.
Nav1.5 is responsible for the initiation of the cardiac action potential. Certain regulatory proteins and post-translational modifications can regulate membrane trafficking and the electric property of Nav1.5 [3]. Many inherited cardiac arrhythmias were caused by abnormal Nav1.5 [4]. For example, long QT syndrome type 3, Brugada syndrome, atrial fibrillation, and congenital sick sinus syndrome are thought to result from the genetic mutations in the Nav1.5 gene (SCN5A). The expression level of Nav1.5 is through to account for some acquired cardiac disorders like heart failure. So far, the molecular mechanisms underlying these disorders are not well understood.
On the other hand, Nav1.7 plays a crucial role in pain syndromes. Mutations that inactivate Nav1.7 function result in the loss-of-pain syndromes in patients who act normally in other aspects [5]. Other mutations in Nav1.7 lead to hyper-active channels and such patients suffer excessive pain syndromes Generally, terminals of sensory neurons highly express Nav1.7 channels, which also supports their pain-sensitive function. The Nav1.7 channels have relatively faster activation and inactivation, but slower recovery, thus even when depolarizations are very small, they can be activated and amplify ramp stimuli to reach the action potential threshold of the neuron [6].
The presence of β subunits facilitates the switch of activation, inactivation, and closed states [7], but the exact mechanism of the gating regulation of β subunits remains largely unexplored. One possible effect that has been proposed is that β subunit Ig domains might bind to the α subunit where they can influence the electric field of VSMs [8]. In addition, the β subunit also has functions independent of the α subunit, such as cell adhesion [9], gene regulation [10] and brain development [11].
The membrane potential in non-excitable cells
As the membrane potential is critical for the electrophysiological signaling in the body, most of the studies focus on its effect on the propagation of action potentials in excitable tissues, such as muscle and nerve. However, the membrane potential depends on the ion channels on the membrane and ion concentrations inside and outside of the cells, which are not unique for excitable cells. The membrane potential was thought to be involved in many biological processes in non-excitable cells [12]. A study showed that the resting membrane potential of cancer cells was about -5 to -52 mV, as a comparison, that of highly proliferating non-cancer cells was about -5 to -25 mV and that of other non-cancer cells was about -95 to -40 mV [13]. Thus, cancer cells have relatively higher resting membrane potentials and these potentials are too positive for Na channels to have a ‘classical’ excitable function.
It has long been found that higher membrane potentials facilitate the proliferation of cells, which is believed to be associated with the initiation of mitosis and DNA synthesis [14,15]. Cancer cells usually have higher membrane potentials compared to their normal counterparts [13], which might be a reason for their fast proliferation. Interestingly, some tumor tissues have a higher level of sodium ions compared to non-cancer tissues, whereas their potassium ions level was similar [16-18], which implies that the intracellular sodium ions level could be the determinant of the abnormal membrane potentials in cancer cells. Therefore, sodium permeable channels might play a critical role in cancers.
The expression of Nav channels in cancers
Several types of channels involving sodium ions transportation have been found different in cancer [19]. One of the most reported channel types is Nav channels. Some of the known expression of Nav channel α subunits in cancers were summarized in Table 1 [20]. Functional Nav channel expression has also been reported in some cancer types, for example, in gastric cancer cell line BGC-823, Nav channel currents were measured by patch-clamp experiments [21]. Nav1.5 and Nav1.7 are the two most expressed Nav channels in human cancers, while Nav1.2 and Nav1.6 are also expressed in some cancer types, such as Mesothelioma [20]. Notably, some cancers express Nav channels neonatal splice variants, which are shown to be more adaptable to the acidified cancer microenvironment [22].
Potential mechanism of Nav channels in cancers
The activation of Nav channels is thought to be involved in some cancer metastatic cascades, including directional motility, pH balance, extracellular proteolysis, and invasion [23]. However, the mechanisms of Nav channels in cancer remain largely unexplored. So far, there are several mechanistic speculations for the role of Nav channels in cancer, which requires further validation and study.
Sodium and other ions homeostasis
Nav channels the sodium–hydrogen antiporter 1 (NHE1, a hydrogen ion channel co-expressed with Nav channels) increase intracellular alkalization and extracellular acidity. The acidic microenvironment of cancer cells promotes the degradation of the extracellular matrix by cysteine cathepsins [24], thereby facilitating cancer cells to leave the primary site [25-27]. On the other hand, sodium ions influx can activate calcium channels in cancer cells [28], which raises the intracellular concentration of calcium ions. This results in more uptake of calcium ions by mitochondria and further leads to a release of calcium ions into the cytosol [29], thereby promoting the formation of the invadopodia, the “feet of cancer cells”, which can elongate the cancer cell to a morphology that facilitates cell movement. This hypothesis is largely based on the observation of Nav channels in macrophage and microglial podosomes [30]. In addition, the formation of invadopodia was also thought to result from Src kinase and cortactin phosphorylation involving cytoskeletal changes [31].
The β subunits
As Ig family cell adhesion molecules, β subunits are proposed to regulate cell adhesion, but studies have shown that β subunit subtypes regulated cancer migration and invasion in different ways. In breast cancer cells, β1 expression was negatively associated with cancer metastasis [32], while in prostate cancer, overexpression of β2 caused an increase in cancer migration and invasion [33]. The expression of β4 was reported to be downregulated in breast cancer cells compared to the non-cancer epithelial cells. Reduced β4 can promote migration and invasion while overexpressed β4 did the opposite [34]. Moreover, a recent study revealed that β3 can bind to tumor suppressor p53 and facilitate the degradation of p53 protein in liver cancer [35]. Although the effects of the β subunit on cancers have been observed, the mechanisms underlying the effects remain largely unknown.
Humoral regulation
Nav channels were through to be involved in the growth factors regulation in cancers. Epidermal growth factor (EGF) was reported to promote the migration and invasion of prostate and non-small cell lung cancer cells by increasing Nav 1.7 expression [36-38]. The regulatory role of nerve growth factor (NGF) in prostate cancer was also found to be associated with the up-regulation of Nav 1.7 [39,40]. Furthermore, the growth factors affecting Nav channels in non-cancer cells might play the same role in cancer cells. For instance, vascular endothelial growth factor (VEGF), a key regulator for cancer angiogenesis [41], has been found to increase Nav channels expression in the DRG neurons [42], while in cardiac myocytes and fibroblasts, the inhibition of Nav1.5 can up-regulate transforming growth factor-beta 1 (TGF-β1), a critical regulator in cancer [43]. Besides, studies have shown that Nav channels are closely associated with the secretion of hormones. In cardiomyocytes, insulin response elements in the SCN5A promoter region can affect the expression of Nav1.5 [44]. In adrenal chromaffin cells and breast cancer cells, insulin was also reported to regulate Nav channels expression [45,46]. Interestingly, the expression of functional Nav channels was found to be potentially associated with the expression of estrogen receptor (ER) in breast cancer cells [47] and basal expression of androgen receptor (AR) in prostate cancer cells [48].
Summary
Voltage-gated sodium channels (Nav channels) are transmembrane proteins that allow the passing of sodium ions through the cell membranes. Nav channels are a critical component of the system that initiates the action potential. Nav channel α and β subunits expression has been found abnormal in cancers. Some studies also indicated the non-classical roles of Nav channels in some cancer types. However, the roles of Nav channel proteins in cancers are still unclear. Cancer cells generally have a relatively higher resting membrane potentials than non-cancer cells. The two most expressed Nav channels in human cancers are Nav1.5 and Nav1.7, while some cancer types also expressed a lower level of Nav1.2 and Nav1.6. There are several mechanistic speculations for the role of Nav channels in cancer, which include sodium-other ions homeostasis, the β subunits, and humoral regulation. To be mentioned, the review includes some personal perspectives that are required for further validation. This review will contribute to the understanding of Nav channels in cancers.
- Catterall WA (2017) Forty Years of Sodium Channels: Structure, Function, Pharmacology, and Epilepsy. Neurochem Res 42: 2495-2504. Link: https://bit.ly/34UDHDx
- de Lera Ruiz M, Kraus RL (2015) Voltage-Gated Sodium Channels: Structure, Function, Pharmacology, and Clinical Indications. Journal of medicinal chemistry 58: 7093-7118. Link: https://bit.ly/3jXaSMJ
- Marionneau C, Abriel H (2015) Regulation of the cardiac Na+ channel NaV1. 5 by post-translational modifications. J Mol Cell Cardiol 82: 36-47. Link: https://bit.ly/37aX2To
- Han D, Tan H, Sun C, Li G (2018) Dysfunctional Nav1.5 channels due to SCN5A mutations. Experimental biology and medicine (Maywood, N.J.) 243: 852-863. Link: https://bit.ly/3k207sI
- Emery EC, Luiz AP, Wood JN (2016) Nav1.7 and other voltage-gated sodium channels as drug targets for pain relief. Expert opinion on therapeutic targets 20: 975-983. Link: https://bit.ly/2GNF9j6
- Herzog RI, Cummins TR, Ghassemi F, Dib‐Hajj SD, Waxman SG (2003) Distinct repriming and closed‐state inactivation kinetics of Nav1. 6 and Nav1. 7 sodium channels in mouse spinal sensory neurons. J Physiol 551: 741-750. Link: https://bit.ly/313sUFT
- Brackenbury WJ, Isom LL (2011) Na+ channel β subunits: overachievers of the ion channel family. Front Pharmacol 2: 53. Link: https://bit.ly/2GNFfqY
- Namadurai S, Yereddi NR, Cusdin FS, Huang CL, Chirgadze DY, et al. (2015) A new look at sodium channel β subunits. Open Biol 5: 140192. Link: https://bit.ly/310Atgs
- Brackenbury WJ (2012) Voltage-gated sodium channels and metastatic disease. Channels (Austin, Tex.) 6: 352-361. Link: https://bit.ly/3110WdF
- Winters JJ, Isom LL (2016) Developmental and Regulatory Functions of Na(+) Channel Non-pore-forming β Subunits. Curr Top Membr 78: 315-351. Link: https://bit.ly/33Xtzul
- Hull JM, Isom LL (2018) Voltage-gated sodium channel β subunits: The power outside the pore in brain development and disease. Neuropharmacology 132: 43-57. Link: https://bit.ly/2GZWiWu
- Abdul Kadir L, Stacey M, Barrett-Jolley R (2018) Emerging Roles of the Membrane Potential: Action Beyond the Action Potential. Front Physiol 9: 1661. Link: https://bit.ly/33Tq8F1
- Yang M, Brackenbury WJ (2013) Membrane potential and cancer progression. Front Physiol 4: 185. Link: https://bit.ly/2H4hpXo
- Orr CW, Yoshikawa-Fukada M, Ebert JD (1972) Potassium: effect on DNA synthesis and multiplication of baby-hamster kidney cells: (cell cycle-membrane potential-synchronization-transformation). Proc Natl Acad Sci U S A 69: 243-247. Link: https://bit.ly/2SUz2vK
- Binggeli R, Weinstein RC (1986) Membrane potentials and sodium channels: hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. J Theor Biol 123: 377-401. Link: https://bit.ly/2GV1xHa
- Smith NR, Sparks RL, Pool TB, Cameron IL (1978) Differences in the intracellular concentration of elements in normal and cancerous liver cells as determined by X-ray microanalysis. Cancer Res 38: 1952-1959. Link: https://bit.ly/3iW3lws
- Cameron IL, Smith NK, Pool TB, Sparks RL (1980) Intracellular concentration of sodium and other elements as related to mitogenesis and oncogenesis in vivo. Cancer Res 40: 1493-1500. Link: https://bit.ly/33YpSVn
- Sparks RL, Pool TB, Smith NK, Cameron IL (1983) Effects of amiloride on tumor growth and intracellular element content of tumor cells in vivo. Cancer Res 43: 73-77. Link: https://bit.ly/3lHJYcm
- Leslie TK, James AD, Zaccagna F, Grist JT, Deen S, et al. (2019) Sodium homeostasis in the tumour microenvironment. Biochimica et biophysica acta. Reviews on cancer 1872: 188304. Link: https://bit.ly/2GV1ACQ
- Djamgoz M, Fraser SP, Brackenbury WJ (2019) In Vivo Evidence for Voltage-Gated Sodium Channel Expression in Carcinomas and Potentiation of Metastasis. Cancers 11: 1675. Link: https://bit.ly/3nNp7Gh
- Xia J, Huang N, Huang H, Sun L, Dong S, et al. (2016) Voltage-gated sodium channel Nav 1.7 promotes gastric cancer progression through MACC1-mediated upregulation of NHE1. Int J Cancer 139: 2553-2569. Link: https://bit.ly/2SUsQUy
- Onkal R, Fraser SP, Djamgoz MB (2019) Cationic Modulation of Voltage-Gated Sodium Channel (Nav1. 5): Neonatal Versus Adult Splice Variants—1. Monovalent (H+) Ions. Bioelectricity 1: 139-147. Link: https://bit.ly/3iTylxj
- Angus M, Ruben P (2019) Voltage gated sodium channels in cancer and their potential mechanisms of action. Channels 13: 400-409. Link: https://bit.ly/2SPqqX6
- Vidak E, Javoršek U, Vizovišek M, Turk B (2019) Cysteine Cathepsins and their Extracellular Roles: Shaping the Microenvironment. Cells 8: 264. Link: https://bit.ly/313hmT9
- Gillet L, Roger S, Besson P, Lecaille F, Gore J, et al. (2009) Voltage-gated Sodium Channel Activity Promotes Cysteine Cathepsin-dependent Invasiveness and Colony Growth of Human Cancer Cells. J Biol Chem 284: 8680-8691. Link: https://bit.ly/379Jqbn
- Busco G, Cardone RA, Greco MR, Bellizzi A, Colella M, et al. (2010) NHE1 promotes invadopodial ECM proteolysis through acidification of the peri‐invadopodial space. FASEB J 24: 3903-3915. Link: https://bit.ly/3k0DViO
- Brisson L, Gillet L, Calaghan S, Besson P, Le Guennec JY, et al. (2011) Na V 1.5 enhances breast cancer cell invasiveness by increasing NHE1-dependent H+ efflux in caveolae. Oncogene 30: 2070-2076. Link: https://go.nature.com/3iXLCEM
- Tajada S, Villalobos C (2020) Calcium Permeable Channels in Cancer Hallmarks. Front Pharmacol 11: 968. Link: https://bit.ly/373TAu3
- Yang M, Kozminski DJ, Wold LA, Modak R, Calhoun JD, et al. (2012) Therapeutic potential for phenytoin: targeting Na(v)1.5 sodium channels to reduce migration and invasion in metastatic breast cancer. Breast Cancer Res Treat 134: 603-615. Link: https://bit.ly/33Tlff3
- Carrithers MD, Chatterjee G, Carrithers LM, Offoha R, Iheagwara U, et al. (2009) Regulation of podosome formation in macrophages by a splice variant of the sodium channel SCN8A. J Biol Chem 284: 8114-8126. Link: https://bit.ly/351Xr8m
- Brisson L, Driffort V, Benoist L, Poet M, Counillon L, et al. (2013) NaV1. 5 Na+ channels allosterically regulate the NHE-1 exchanger and promote the activity of breast cancer cell invadopodia. J Cell Sci 126: 4835-4842. Link: https://bit.ly/3lBRNAm
- Chioni AM, Brackenbury WJ, Calhoun JD, Isom LL, Djamgoz MB (2009) A novel adhesion molecule in human breast cancer cells: voltage-gated Na+ channel beta1 subunit. Int J Biochem Cell Biol 41: 1216-1227. doi:10.1016/j.biocel.2008.11.001. Link: https://bit.ly/34Oub4w
- Jansson KH, Lynch JE, Lepori‐Bui N, Czymmek KJ, Duncan RL, et al. (2012) Overexpression of the VSSC‐associated CAM, beta‐2, enhances LNCaP cell metastasis associated behavior. Prostate 72: 1080-1092. Link: https://bit.ly/2H0xuxq
- Bon E, Driffort V, Gradek F, Martinez-Caceres C, Anchelin M, et al. (2016) SCN4B acts as a metastasis-suppressor gene preventing hyperactivation of cell migration in breast cancer. Nature Communications 7: 1-18. Link: https://go.nature.com/3dslyAw
- Li S, Han J, Guo G, Sun Y, Zhang T, et al. (2020) Voltage-gated sodium channels beta3 subunit promotes tumorigenesis in hepatocellular carcinoma by facilitating p53 degradation. FEBS letters 594: 497-508. Link: https://bit.ly/3nNyMN4
- Campbell TM, Main MJ, Fitzgerald EM (2013) Functional expression of the voltage-gated Na⁺-channel Nav1.7 is necessary for EGF-mediated invasion in human non-small cell lung cancer cells. J Cell Sci 126: 4939-4949. Link: https://bit.ly/34Ts1kd
- Ding Y, Brackenbury WJ, Onganer PU, Montano X, Porter LM, et al. (2008) Epidermal growth factor upregulates motility of Mat-LyLu rat prostate cancer cells partially via voltage-gated Na+ channel activity. J Cell Physiol 215: 77-81. Link: https://bit.ly/3nNyYfg
- Uysal-Onganer P, Djamgoz MB (2007) Epidermal growth factor potentiates in vitro metastatic behaviour of human prostate cancer PC-3M cells: involvement of voltage-gated sodium channel. Molecular Cancer 6: 76. Link: https://bit.ly/33TlJ4R
- Brackenbury WJ, Djamgoz MB (2007) Nerve growth factor enhances voltage-gated Na+ channel activity and Transwell migration in Mat-LyLu rat prostate cancer cell line. J Cell Physiol 210: 602-608. Link: https://bit.ly/3lMJ91M
- Diss JK, Calissano M, Gascoyne D, Djamgoz MB, Latchman DS (2008) Identification and characterization of the promoter region of the Nav1. 7 voltage-gated sodium channel gene (SCN9A). Mol Cell Neurosci 37: 537-547. Link: https://bit.ly/3nRhWwK
- Goumans MJ, Ten Dijke P (2018) TGF-β Signaling in Control of Cardiovascular Function. Cold Spring Harbor perspectives in biology 10. Link: https://bit.ly/2GWaX5d
- Malykhina AP, Lei Q, Erickson CS, Epstein ML, Saban MR, et al. (2012) VEGF induces sensory and motor peripheral plasticity, alters bladder function, and promotes visceral sensitivity. BMC Physiol 12: 15. doi:10.1186/1472-6793-12-15. Link: https://bit.ly/2GZmXmp
- Colak S, Ten Dijke P (2017) Targeting TGF-β Signaling in Cancer. Trends Cancer 3: 56-71. Link: https://bit.ly/3nNl0Kv
- Mao W, You T, Ye B, Li X, Dong HH, et al. (2012) Reactive oxygen species suppress cardiac NaV1.5 expression through Foxo1. PLoS One 7: e32738. Link: https://bit.ly/33Tm3k5
- Nemoto T, Yanagita T, Kanai T, Wada A (2009) Drug development targeting the glycogen synthase kinase-3beta (GSK-3beta)-mediated signal transduction pathway: the role of GSK-3beta in the maintenance of steady-state levels of insulin receptor signaling molecules and Na(v)1.7 sodium channel in adrenal chromaffin cells. J Pharmacol Sci 109: 157-161. Link: https://bit.ly/2GNiaoh
- Pan H, Djamgoz MB (2008) Biochemical constitution of extracellular medium is critical for control of human breast cancer MDA-MB-231 cell motility. J Membr Biol 223: 27-36. Link: https://bit.ly/36YHhiy
- Fraser SP, Diss JK, Chioni AM, Mycielska ME, Pan H, et al. (2005) Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res 11: 5381-5389. Link: https://bit.ly/3lPZgvT
- Diss JK, Archer SN, Hirano J, Fraser SP, Djamgoz MB (2001) Expression profiles of voltage‐gated Na+ channel α‐subunit genes in rat and human prostate cancer cell lines. Prostate 48: 165-178. Link: https://bit.ly/34ZCxGC

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
