Global Journal of Cancer Therapy
Video Endoscopic Inguinal Lymphadenectomy: Refining surgical technique after ten years experience
Marcos Tobias-Machado1, Pericles Rios Auad1*, Victor Corona2, Igor Silva6, Oseas de Castro Neves1, Eliney Ferreira Faria5, Pablo Matos3, Alexandre Cesar Santos5, Roberto Machado5, Aurus Dourado4 and Hamilton de Campos Zampolli6
2Hospital General de Ciudad del Mexico, Mexico
3São Marcos Hospital, Teresina, Brazil
4Recife Cancer Hospital, Pernambuco, Brazil
5Barretos, Cancer Hospital, São Paulo, Brazil
6Dr. Arnaldo Vieira de Carvalho, Cancer Institute, São Paulo, Brazil
Cite this as
Tobias-Machado M, Auad PR, Corona V, Silva I, de Castro Neves O, et al. (2017) Video Endoscopic Inguinal Lymphadenectomy: Refining surgical technique after ten years experience. Glob J Cancer Ther 3(1): 034-037. DOI: 10.17352/gjct.000021Introduction
Penile carcinoma is a rare malignant disease with a significantly higher incidence in some areas of under- developed countries [1]. Inguinal nodal involvement is found in 20% to 40% of cases at diagnosis and nodal metastasis is an important predictive factor for survival [2,3]. Metastatic penile carcinoma has an extremely poor prognosis, since reported results of systemic therapy have been disappointing, even when improvement is achieved [4-12].
Inguinal lymphadenectomy is indicated in patients presenting penile and urethral cancer, after local treatment, when there is a lymph node mass that does not disappear with antibiotic therapy or when palpable lymph nodes appear in the postoperative follow-up or when there are risk factors for the development of inguinal metastasis (prophylactic lymphadenectomy). This operation has been frequently performed through a bilateral inguinal incisions from the iliac crest until the pubic tubercle. For some years now, Video Endoscopic Inguinal Lymphadenectomy (VEIL) has been an alternative less invasive and with the same oncologic results of the open surgery [6,7].
Our group has performed this procedure for 10 years and in this time frame we have changed some points. We aim to describe the changes in the VEIL technique (called from now on as refining VEIL) compared to the initial description [1].
Methods
The technique described was developed and performed in 20 procedures in 12 patients presenting penile carcinoma without palpable lymph nodes > 2 cm (non-palpable and low volume palpable nodes). All patients had an indication of bilateral lymphadenectomy due to the presence of risk factors for lymph node dissemination as: clinical stage > T1 or information regarding the initial biopsy such as histological grade > 1, lymphatic or vascular invasion.
When simultaneous bilateral procedure was feasible (2 skill surgeons and 2 laparoscopic sets were available), it was the chosen method.
We evaluate operative time, complication, number of lymph nodes, hospital stay and oncological results compared to other 20 cases of conventional VEIL.
Surgical Technique
Conventional VEIL
The technique was performed as in a previous study of our group [1-4].
Refining VEIL
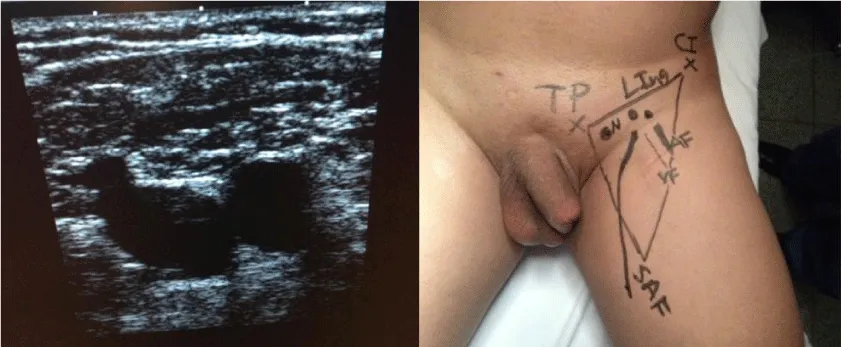
1 – Before performing the procedure, an ultrasound of the inguinal region was done and we ink marked the detected lymph nodes and saphenous vein [4] (Figure 1).
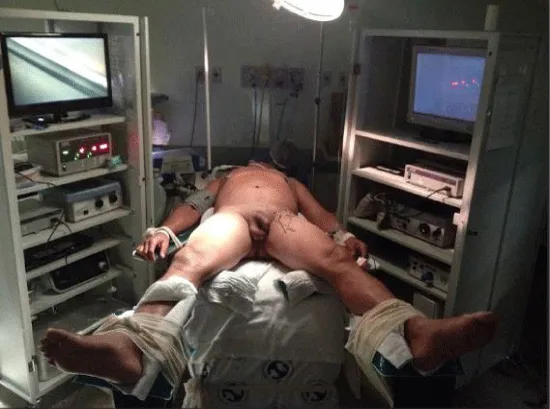
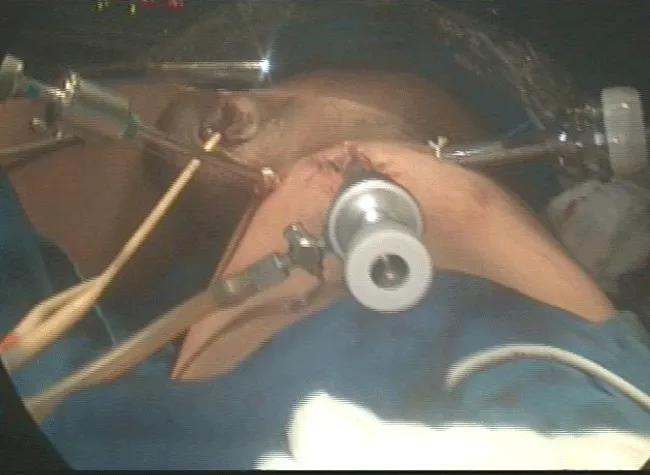
2 – Positioning and preparation of the inferior member – The leg was folded over the thigh in a way to put in evidence the femoral triangle that is marked with ink over the skin. After the marking, the leg is extended and fixed to the table with abduction and light external rotation of the thigh. The monitor is positioned at the contralateral side at patient’s pelvic waist (Figure 2).
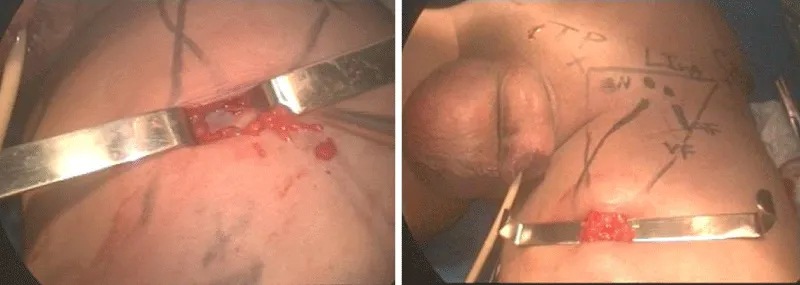
3 – At the femoral triangle vertex is performed a 2 cm incision. Helped by Farabeuf retractor is possible to dissect the lymph node block at vertex over fascia lata using Sartorius muscle and tight adductor fascias as reference. A non-absorbable ligation and division is made at this point. In most cases, we can access the greater saphenous vein through this incision that can be ligated if necessary (Figure 3).
4 – With a digital dissection is possible to separate the lymph node block from fascia lata for at least 5 cm proximally.
5 – It is performed a dissection under the Scarpa`s fascia as far as possible avoiding digital dissection only between lateral ports position (area of nodes and saphena branches) (Figure 4).
6 – Two ports are positioned 2 cm distally and 6 cm laterally and medially to the initial incision. The lateral port is 10 mm and medial port is 5 mm.
7 – Initial incision closure for sealing a 10 mm port where is positioned at 0 degree scope.
8 – Expansion with gas of the working space with a 15 mmHg pressure (Figure 5).
9 – Retrograde separation of the skin flap with harmonic scalpel. Initially, we perform a separation between the skin and the fibroareolar tissue that contains the superficial lymph nodes until the external oblique muscle fascia on the superior part. Afterwards we proceed to the dissection of the fundamental parameters, having as a limit the long adductor muscle and its fascia medially, the Sartorius muscle and its fascia laterally, and the inguinal ligament superiorly. It is possible to identify branches of the femoral nerve that should be preserved.
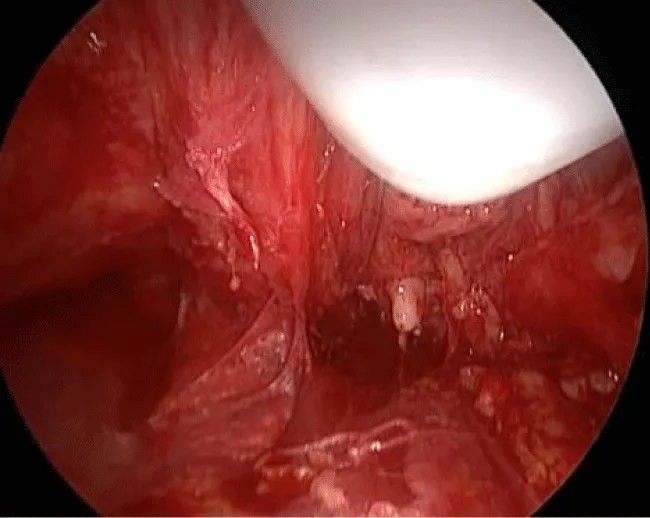
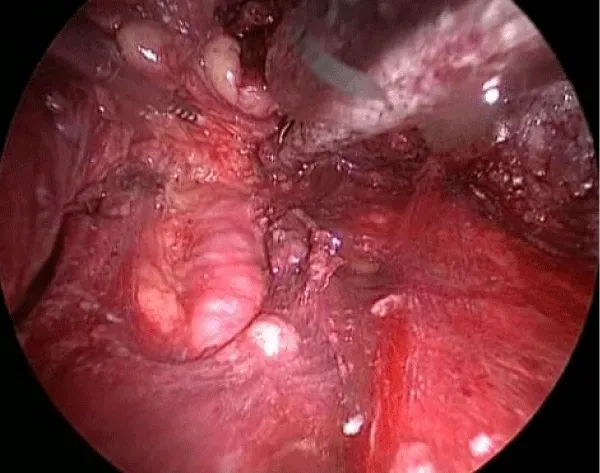
10 – Identification and cranial dissection of long saphenous vein previously ligated until the oval fossa (Figure 6).
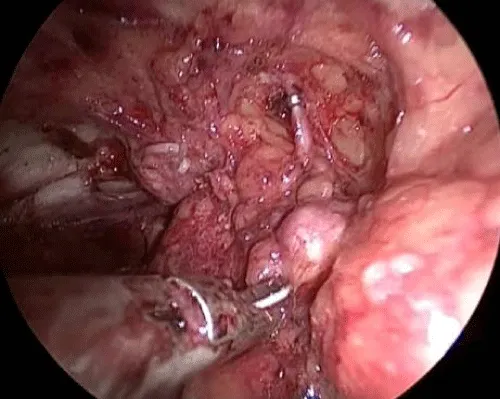
11 – Identification of the femoral vessels. After the identification of the femoral artery and the opening of the femoral vein sheath we define the lateral limit of the dissection, allowing the access to the deep lymph nodes (Figure 7).
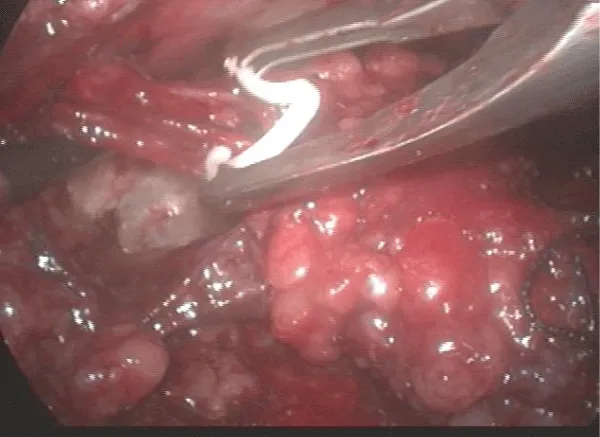
12 – Liberation of the lymph nodes until the great vessels above the femoral floor. During this operative time, the use of the harmonic scalpel and a careful manipulation of the specimen in areas near the veins are necessary to avoid vascular lesions. All lymphatic vessel identified may be controlled with clips to avoid excessive lymphatic drainage. The aim is to skeletonize the femoral veins, resecting all local lymphatic tissue (Figure 8).
13 – Control of the branches and the long sapheno-femoral junction with a harmonic scalpel and metallic clips - branches larger than 4 mm need clips for the ligature.
14 – Final liberation of the specimen medially to the long saphenous vein, ligating the proximal portion of the lymph nodes at the deep region of the femoral channel with clips. After completing the liberation of the specimen, the endoscope view attests that all the tissue of the region was completely resected. If available biologic glue can be applied over this resected area.
15 - Removal of the surgical specimen by the 20 mm incision.
16 – Deflate the gas and withdrawal the instruments.
17 – Intraoperative ultrasound to make sure that all the lymphatic tissue was removed.
18 – Vacuum drainage through the 5 mm orifice and suture of the larger incisions.
Changes from the initial technique
Changes proposed to our initial VEIL description aimed to simplify the apical triangle dissection, maximizing the control of lymphatic channels and promoting an improved control of lymph nodes resection.
Preoperative ultrasound was introduced before the procedure in order to identify and mark enlarged lymph nodes. After resection, an intraoperative ultrasound was performed after deflation to check if there is any residual affected tissue.
Initially, the first port was positioned 2 cm distal to the femoral triangle vertex and now it is inserted right on the vertex. The distal limit of the lymph node block is ligated by this initial incision and digital dissection, as proximal as possible, was performed.
The most important improvement was the optimization of clip use, instead of ultrasonic energy to control small lymphatics.
In patients with indication of both groins endoscopic dissection, we recommend bilateral simultaneous procedure in order to reduce total operative time.
Results
We included patients operated by a master surgeon or one trainee assisted by the master from 5 different institutions. We perform “refining VEIL” new technique in 20 groins performed in 12 patients (group 1). This data was compared to the last 20 operated groins of patients under the same indicative criteria (group 2).
The most important data is presented at table 1. Both groups are demographically similar. We found a significant reduction of operative time from 100 to 65 minutes (p = 0.03) on each side.
Perioperative complications were similar. Skin events were extremely low in both groups. Lymphatic events were smaller in “refining VEIL” without statistical significance. The number of removed lymph nodes was also similar. Mean time to remove drainage was smaller in “refining VEIL” (p=0.04
Preliminary oncological results were comparable.
Discussion
VEIL is an emerging minimally invasive surgical option to inguinal dissection recently reproduced in small series by several centers in America, Asia and Europe.
All of reported series shows an impressive reduction of skin morbidity compared to open surgery (0-2% versus 20-30%) [8]. Reduction of lymphatic events was not demonstrated in this preliminary reports due to the small number of related events which are probably explained by the relatively low practice and the indiscriminate use of energy to seal lymphatic vessels [8-13]. To improve this aspect, we indicate to increase the use of clipping to control small vessels, visualized by laparoscopic magnified vision. As an adjuvant maneuver, biological glue can be used to reduce lymphatic drainage, as demonstrated in other studies of groin dissection [9-13].
One of the challenges at the initial technique description was to work on the femoral triangle vertex. Ergonomy and angulation of the instruments with skin (close to 90 degrees) were hard to perform and took time. We used to ligate the saphenous vein and lymphatic tissue with hem-o-lock at this point. Based on the technical changes introduced, we perform this steps openly, avoiding skin detachment, saving time, decreasing CO2 exposure and reducing costs.
Other potential criticism of VEIL was the long total operative time, when bilateral sequential procedure were performed (3-4h). In order to reduce operative time, an initial external approach of vertex and bilateral simultaneous procedure can be used with excellent results.
Oncological outcomes were poorly reported but at least 2 centers show that long term oncological results were exactly the same as those reported by open series [6,7]. Furthermore, a report tried to prove oncological adequacy of VEIL in cadaveric model and patients by performing an open incision after VEIL to verify if a lymph node was left in the planned dissected area [7]. Both studies shows that VEIL can remove the same area of an opened inguinal dissection [7,8]. In order to improve control of the resected area, we indicate the use of ultrasound image in the preoperative settings in order to identify the number and position of affected lymph nodes. At the end of the procedure, ultrasound image can confirm if all lymphatic tissue previously defined were completely surgically removed.
Recently, some reports of robotic VEIL emerged with good results [10]. Excellent ergonomics and magnification are the supposed benefits. We believe that proposed modifications can be applied to robotic surgery as well. We speculate that, in the near future, robotic platform can improve lymphatic identification by using green fluorescence, as in other procedures [4].
Conclusion
VEIL is a reproducible technique that has been widely used for many years since its development.
Our experience led us to make a few changes in the technique, now called refining VEIL, with benefits, such as operative time shortening, lower cost, potential reduced morbidity and the certainty of complete tissue removal, keeping and improving oncological safety.
- Tobias-Machado M, Starling ES, Oliveira ABP, Pompeo AC, Wroclawski ER (2009) 5-years experience with Video Endoscopic Inguinal Lymphadenectomy (VEIL): learning curve and technical variations of a new procedure. Journal of Andrological Sciences 16: 25–32. Link: https://goo.gl/F6YQ5D
- Pompeo A, Tobias MM, Molina WR, Lucio J, Sehrt D, et al. (2013) Extending boundaries in minimally invasive procedures with simultaneous bilateral video endoscopic inguinal lymphadenectomy (veil) for penile cancer: initial Denver health medical center and ABC school of medicine experience and surgical considerations. Int Braz J Urol 39: 587-592. Link: https://goo.gl/U7XZJm
- Ornellas AA, Seixas AL, Marota A, Wisnescky A, Campos F, et al. (1994) Surgical treatment of invasive squamous cell carcinoma of the penis: retrospective analysis of 350 cases. J Urol 151: 1244-1249. Link: https://goo.gl/K7ibzs
- M Tobias-Machado, Alessandro T, Wilson R Molina Jr, Pedro H Forseto Jr, Roberto V Juliano, et al. (2006) Video Endoscopic Inguinal Lymphadenectomy (VEIL): Minimally Invasive Resection of Inguinal Lymph Nodes. International Braz J Urol 32: 316-321. Link: https://goo.gl/f927ti
- Pompeo ACL, Heyns CF, Abrams P (2009) Penile Cancer – International Consultation on Penile Cancer. Societé Internationale d’Urologie (SIU). Urology.
- Machado MT, Molina Jr WR, Tavares A, Forseto Jr PH, Juliano RV, et al. (2005) Comparative study between videoendoscopic inguinal lymphadenectomy (VEIL) and standart open procedure for penile cancer: preliminary surgical and oncological results. J Urol 173: 22.
- Bishoff JÁ, Lackland AFB, Basler JW, Teichman JM, Thompson IM (2003) Endoscopy subcutaneous modified inguinal limph node dissection (ESMIL) for Squamous cell carcinoma of the penis. J Urol 169: 78.
- Sotelo R, Sánchez SR, Carmona O, Garcia A, Mariano M, et al. (2007) Endoscopic lymphadenectomy for penile carcinoma. J Endouroul 21: 364-367. Link: https://goo.gl/uXabvf
- Gianluca Di Monta, Corrado Caracò, Anna Crispo, Ugo Marone, N Mozzillo (2012) Collagen sealant patch to reduce lymphatic drainage after lymph node dissection World J Surg Oncol 10: 275. Link: https://goo.gl/9uXqnS
- Jain V, Sekhon R, Giri S, Hassan N, Batra K, et al. (2017) Robotic-Assisted Video Endoscopic Inguinal Lymphadenectomy in Carcinoma Vulva: Our Experiences and Intermediate Results. Int J Gynecol Cancer 27: 159-165. Link: https://goo.gl/QRtv6p
- Koifman L, Hampl D, Koifman N, Vides AJ, Ornellas AA (2013) Radical open inguinal lymphadenectomy for penile carcinoma: surgical technique, early complications and late outcomes. J Urol 190: 2086-2092. Link: https://goo.gl/excgnX
- Walsh PC, Retik AB, Vaughan Jr ED, Wein AJ, Kavoussi LR, et al. (2002) Campbell’s Urology, 8.ed. Philadelphia Saunders 2945-2948.
- Tobias B, Koifman (2013)The risks associated with this kind of surgery are the postoperative appearance of minor (wound infection, seroma, leg edema, skin edge necrosis and scrotal edema) and major (wound infection, lymphocele, flap necrosis, wound abscess and deep venous thrombosis) tissue problems, as reported by some authors
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
