Global Journal of Cancer Therapy
Lenvatinib Suppresses Angiogenesis through the Inhibition of both the VEGFR and FGFR Signaling Pathways
Kenji Ichikawa, Saori Watanabe Miyano, Yusuke Adachi, Masahiro Matsuki, Kiyoshi Okamoto and Junji Matsui*
Cite this as
Ichikawa K, Miyano SW, Adachi Y, Matsuki M, Okamoto K, et al. (2016) Lenvatinib Suppresses Angiogenesis through the Inhibition of both the VEGFR and FGFR Signaling Pathways. Glob J Cancer Ther 2(1): 019-025. DOI: 10.17352/gjct.000009Lenvatinib mesilate (lenvatinib) is an oral multiple-receptor tyrosine kinase inhibitor that selectively inhibits the kinase activities of Vascular Endothelial Growth Factor Receptor (VEGFR) 1-3, Fibroblast Growth Factor Receptor (FGFR) 1-4, Platelet-Derived Growth Factor Receptor (PDGFR) α, KIT, and RET. The VEGFR and FGFR signaling pathways are the master regulators of normal and tumor angiogenesis. Lenvatinib showed significant activity in patients with radioiodine-refractory thyroid cancer in a Phase III study and is used in the United States, the European Union, and Japan. Moreover, based on Phase II study, lenvatinib has been approved in the United States for the treatment of patients with advanced renal cell carcinoma in combination with everolimus. In addition, the efficacy of lenvatinib is being evaluated in other cancers, including hepatocellular carcinoma and endometrial cancer. The purpose of this study was to elucidate the mechanism underlying the clinical activities of lenvatinib by using in vitro and in vivo angiogenesis models.
First, we established an in vitro tube formation system, in which capillary-like structures formed on basement membrane extract in response to pro-angiogenic factors. Lenvatinib suppressed tube formation induced by bFGF alone and by bFGF plus VEGF. Furthermore, plasma levels of VEGF and FGF23, pharmacodynamic biomarkers of inhibition of the VEGFR and FGFR signaling pathways, respectively, were up-regulated after the administration of lenvatinib to mice. By contrast, the administration of another VEGFR inhibitor, sorafenib tosylate (sorafenib), up-regulated plasma levels of VEGF but not FGF23. Finally, lenvatinib suppressed bFGF-driven angiogenesis in Matrigel plug assays at low dosage (3 mg/kg), whereas sorafenib did so only at a higher dose (30 mg/kg). These results indicate that lenvatinib inhibits both VEGFR and FGFR in vitro and in vivo. This combined inhibition of both VEGFR and FGFR may lead significant clinical activities.
Introduction
Angiogenesis, the formation of new blood vessels from the pre-existing vasculature, is required for embryonic development, wound repair, and tumor growth [1]. In tumors, angiogenesis is necessary to supply oxygen and nutrients to proliferating cancer cells. Pro-angiogenic factors such as VEGF and FGF promote the migration, proliferation, differentiation, and eventually survival of endothelial cells to form tumor vessels [2-4].
The same newly formed vessels that transport growth factors, cytokines, and nutrients to cancer cells provide the route for cancer metastasis. Consequently, the activation of tumor angiogenesis correlates with both the growth of the tumor mass and cancer metastasis. The VEGF–VEGFR axis plays an integral role in tumor angiogenesis [5]. VEGF activates VEGFR through homo- and heterodimerization by interacting with the extracellular domain of VEGFR on endothelial cells [6,7]. The VEGF–VEGFR signaling pathway also plays a critical role in promoting endothelial cell growth and migration and thus tumor vascularization. For these reasons, many inhibitors of the VEGFR signaling pathway, such as bevacizumab, sorafenib, and sunitinib, have been used as therapeutic agents in several tumor types, including renal cell carcinoma, non-small cell lung carcinoma, hepatocellular carcinoma, and thyroid cancer [8,9]. These inhibitors successfully suppress tumor angiogenesis and mass in a subset of patients.
The FGF–FGFR axis is another pro-angiogenic signaling pathway [10]. Indeed, high levels of bFGF expression are correlated with a worse prognosis in highly vascularized tumor types, such as renal cell carcinoma and hepatocellular carcinoma [11,12]. Injection of adenovirus encoding soluble FGFR into RIP1-Tag2 mouse showed decrease of vessel density in tumor, leading to growth inhibition [13]. Like VEGF, bFGF induces the proliferation, survival, and differentiation of endothelial cells, ultimately activating tumor angiogenesis. Accordingly, the FGF–FGFR axis is recognized as a potential therapeutic target for blocking tumor angiogenesis.
Biomarkers for molecular-targeted agents are important for assessing drug candidates in clinical trials. For example, validated biomarkers contribute to clinical studies by providing means for predicting the mechanisms of action underlying the efficacies and toxicities of candidate drugs and for determining therapeutic doses. Two biomarkers that reliably reflect the inhibition of the VEGFR and FGFR pathways are blood concentrations of VEGF and FGF23, respectively. Indeed, several VEGFR inhibitors increased plasma VEGF levels in both preclinical and clinical studies [14-16]. FGF23 belongs to the FGF family and functions as an endocrine factor. Increased plasma FGF23 levels by FGFR inhibitors are a surrogate pharmacodynamic biomarker of FGFR inhibition [17].
Lenvatinib is an orally administered multiple receptor tyrosine kinase inhibitor with a novel binding mode that selectively targets the kinase activities of VEGFRs (VEGFR1-3) and FGFRs (FGFR1-4) in addition to other pro-angiogenic and oncogenic pathway-related RTKs including PDGFRα, KIT and RET [18-23]. Our previous study reveals that Ki values of lenvatinib against VEGFRs are between 0.7 and 1.3 nmol/L, and FGFRs are between 8 and 22 nmol/L [19]. Due to the results of a Phase III clinical study, lenvatinib was approved in the United States, European Union, and Japan in 2015 for the treatment of advanced, differentiated thyroid cancer that is refractory to radioactive iodine or unresectable thyroid cancer [24]. Furthermore, lenvatinib has been approved for the treatment of patients with advanced renal cell carcinoma following one prior anti-angiogenic therapy in combination with everolimus in the United States based on Phase II study [25]. Currently lenvatinib is being evaluated for efficacy in various other cancers, including hepatocellular carcinoma and endometrial cancer.
In this study, we show that lenvatinib inhibited angiogenesis in vitro and in vivo through the dual targeting of VEGFR and FGFR. These results suggest that this combined anti-angiogenic activity of lenvatinib contributes to its clinical activities.
Material and Methods
Cells and reagents
Human umbilical vein endothelial cells (HUVECs) were isolated from a human umbilical cord as described previously [19] and cultured in supplemented EBM-2 as provided in the EGM-2 BulletKit (Lonza). The human differentiated thyroid cancer cell line RO82-W-1 was obtained from the European Collection of Authenticated Cell Cultures (ECACC) and cultured in a mixture of DMEM (WAKO), Ham’s F12 (WAKO), and MCDB 105 (Sigma-Aldrich) (2:1:1) supplemented with 10% fetal bovine serum. All cells were maintained at 37°C in a 5% CO2 atmosphere.
Lenvatinib, sorafenib, and PD173074, a selective FGFR inhibitor were synthesized at Eisai Co., Ltd. (Ibaraki, Japan).
In vitro tube formation assay
Geltrex (45 µL/well; Thermo Fisher Scientific) was added to each well of 96-well plates and incubated at 37°C in a 5% CO2 atmosphere for 30 minutes to allow the gel to solidify. Medium 200PRF (Thermo Fisher Scientific) containing bFGF only (80 ng/mL; Invitrogen) or both bFGF (80 ng/mL) and VEGF (80 ng/mL; R&D Systems) was incubated with anti-bFGF antibody (1000 ng/mL; 05-117, MILLIPORE), anti-VEGF antibody (1000 ng/mL; MAB293, R&D Systems), or both anti-bFGF antibody and anti-VEGF antibody at 4°C overnight. HUVECs were diluted to 1.6×105 cells/mL in Medium 200PRF, and 75 µL of this cell suspension was dispensed into each well containing the solidified gel in 96-well plates. Pre-incubated antibody–bFGF or antibody–bFGF–VEGF mixture, lenvatinib solution (0.610−4000 nmol/L) and 80 ng/mL bFGF, lenvatinib solution (0.610−4000 nmol/L) and 80 ng/mL bFGF–VEGF, or vehicle only was then added to each well (25 µL; final concentration of each ligand, 20 ng/mL). The plates were cultured for 20 hours at 4°C, after which 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (100 µL; 3.3 mg/mL in phosphate-buffered saline; Sigma) was added to each well; plates were incubated for an additional 4 hours for the formation of capillary-like structures. Images of tubes were obtained by using a GelCount device (Oxford Optronix), and the length of the tubes (capillaries) was measured by using the In Cell Developer Toolbox (version 1.9.2, GE Healthcare). This experiment was performed three times in triplicate.
Measurement of VEGF in mouse plasma
Cultured RO82-W-1 cells were suspended in Hank’s Balanced Salt Solution (Thermo Fisher Scientific) and mixed with an equal volume of Matrigel (Corning) to yield a suspension containing 5×107 cells/mL. A 0.1-mL aliquot of this cell suspension was transplanted subcutaneously into the right flank region of each BALB/c nude mouse (CAnN.Cg-Foxn1nu/CrlCrlj, female, Charles River Japan). When tumor volume reached approximately 350 mm3, mice were grouped according to tumor volume and shape and randomly allocated to receive vehicle, lenvatinib, or sorafenib (n = 8 per group). Lenvatinib (doses, 3 and 10 mg/kg), sorafenib (dose, 30 mg/kg), or vehicle only (distilled water) were administered orally once daily for 12 days at 0.1 mL/10 g body weight. At 9 hours after the last administration, blood was collected from the abdominal aorta of isoflurane-anesthetized mice. Blood was then centrifuged at 20,000 g for 5 minutes at 4°C, and the plasma was collected. The amount of VEGF in the mouse plasma was measured by using the Mouse VEGF Quantikine ELISA Kit (R&D Systems); this assay was performed in duplicate.
Measurement of FGF23 in mouse plasma
Lenvatinib (doses, 3 and 10 mg/kg), sorafenib (doses, 9 and 30 mg/kg), or vehicle only (distilled water) was orally administered to BALB/c nude mice (CAnNCrlCrlj, female, Charles River Japan) at 0.1 mL/10 g body weight (n = 8 per group). At 24 hours after this single administration, blood was collected from the abdominal aorta of isoflurane-anesthetized mice, centrifuged at 9,000 g for 10 minutes at 4°C, and the plasma collected. The amount of plasma FGF23 was measured by using the FGF-23 ELISA kit (Kainos Laboratories) according to the manufacturer’s procedure. The assay was performed in duplicate.
Matrigel plug assay
BALB/c nude mice (CAnN.Cg-Foxn1nu/CrlCrlj, female, Charles River Japan) were each injected subcutaneously in the abdominal region with 300 µL of Matrigel (Corning) containing 1 µg/mL bFGF (Invitrogen) or without bFGF. Mice injected with bFGF-containing Matrigel were allocated into 6 groups (n=8 per group, Day 1). Lenvatinib and sorafenib were orally administered at 0.1 mL/10 g body weight and PD173074 was orally administered at 0.2 mL/10 g body weight once daily for 7 days (Days 1–7). On Day 8, mice were euthanized, and the Matrigel plug isolated from each mouse was minced in 400 µL of distilled water and placed in the dark at 4°C for 2 days to release any hemoglobin in the plugs into the water. To quantify the formation of neovasculature, the hemoglobin content in the Matrigel plug was measured by using Drabkin’s solution (Sigma). Briefly, the supernatant was isolated by centrifugation and plated in 96-well microtiter plates (100 µL/well), Drabkin’s solution was added at 100 µL/well, and the plate was placed in the dark for 2 hours at room temperature. The optical density of each well was measured at 540 nm (reference wavelength: 660nm) by using a microplate reader (Spectra Max250, Molecular Devices). The hemoglobin content of each plug was calculated by using the value of the hemoglobin standard.
Antitumor activity of lenvatinib and sorafenib in K1 or RO82-W1 xenografts in mice
K1 and RO82-W1 cells were washed with Dulbecco’s phosphate buffered saline, harvested with 0.25% trypsin–EDTA, and suspended with 50% BD Matrigel™ (BD biosciences) in the mixture medium at a density of 5.0 × 107 cells/ml for K1 xenografts model and 2.5 × 107 cells/mL for RO82-W1 xenografts. The cell suspension was inoculated subcutaneously into the right flank region of each mouse. Mice were selected based on their tumor volumes, shapes of tumors, physical condition, and body weights, and were randomly divided into 12 groups (Day 1). Lenvatinib mesilate (1, 3, 10, 30, and 100 mg/kg), sorafenib tosylate (3, 10, 30, 100, and 300 mg/kg), or vehicle was orally administered at 0.2 and 0.1 mL/10 g body weight, respectively, once daily. The tumor volumes were measured on day 15 for K1 xenografts model and on day 22 for RO82-W1 xenografts.
The tumor volume was calculated according to the following formula;
Tumor volume (mm3) = length (mm) × width2 (mm2) × 1/2
Length: largest diameter of tumor
Width: diameter perpendicular to length
The %TGI was calculated according to the following formula;
%TGI = (1 – dT / dC) × 100
dT = Tumor volume on last day – Tumor volume on Day 1
dC = Mean tumor volume of the vehicle-treated group on last day – Mean tumor volume of the vehicle-treated group on Day 1
All procedures using laboratory animals were conducted in accordance with the Institutional Animal Care and Use Committee guidelines of Eisai Co., Ltd.
Statistical analysis
All data are presented as mean ± SD. The differences between the means of groups were analyzed by unpaired t test or one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. A value of P < 0.05 (two sided) was considered statistically significant. Statistical analyses were performed using GraphPad Prism version 6.02.
Results
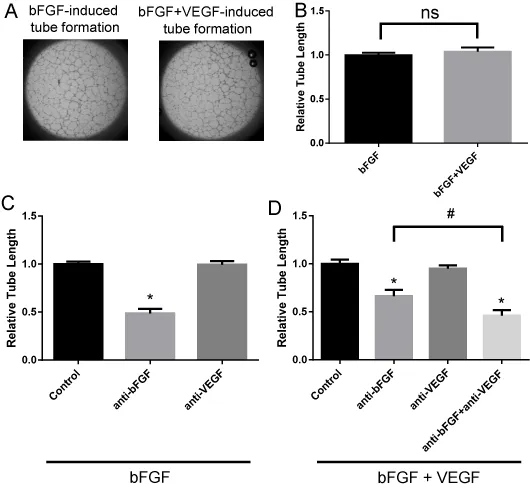
Establishment of bFGF or bFGF plus VEGF-induced tube formation assay
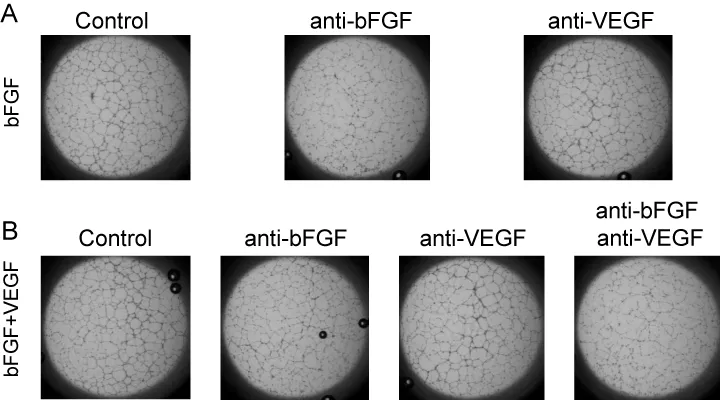
To evaluate the inhibitory activity of lenvatinib against VEGFR and FGFR in angiogenesis in vitro, we established and validated a tube formation assay system in which HUVECs form capillary-like structures on Geltrex (Invitrogen), a basement membrane extract with reduced concentrations of growth factors. First, we tested whether VEGF, bFGF, and the combination of VEGF plus bFGF-induced tube formation on Geltrex. Tube formation was potently induced in response to bFGF and the combination of VEGF plus bFGF (Figure 1A, B) but not to VEGF only (data not shown). To examine ligand specificity in this system, anti-bFGF and anti-VEGF antibodies were used to disrupt the function of the bFGF and VEGF ligands, respectively. Anti-bFGF antibody significantly decreased bFGF-induced tube formation, whereas anti-VEGF antibody did not (Figure 1C, Figure S1A). Furthermore, both anti-bFGF antibody alone and a mixture of anti-bFGF and anti-VEGF antibodies significantly inhibited bFGF- plus VEGF-induced tube formation compared with that of the control, and the inhibitory activity of the antibody mixture was greater than that of anti-bFGF antibody alone (Figure 1D, Figure S1B). Treatment with anti-VEGF antibody alone failed to inhibit the tube formation induced by bFGF plus VEGF (Figure 1D). These results suggest that bFGF acts as strong pro-angiogenic factor in this assay system and that VEGF contributes to tube formation in the presence of bFGF. Additional activity of VEGF in the VEGF-bFGF combination setting did not observed (Figure 1B), probably because 20 ng/ml bFGF achieved complete effect on tube formation.
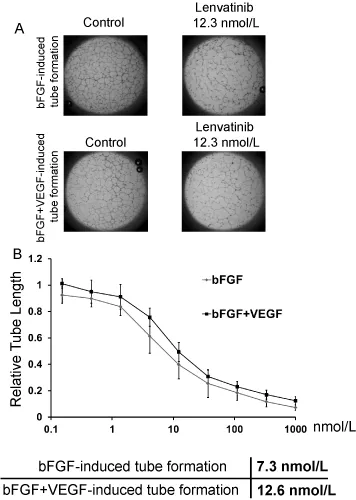
Disruption of bFGF or bFGF plus VEGF-induced tube formation by lenvatinib
Next, we examined the inhibitory activities of lenvatinib against the tube formation induced by bFGF or bFGF plus VEGF in our established assay system. Lenvatinib at 12.3 nmol/L strongly disrupted the tube formation induced by bFGF or by bFGF and VEGF (Figure 2A). Moreover, lenvatinib inhibited tube formation induced by bFGF in a dose-dependent manner with IC50 value of 7.3 nmol/L. In addition, lenvatinib decreased the tube formation induced by bFGF plus VEGF with IC50 value of 12.6 nmol/L (Figure 2B)—that is, at almost the same dosage as that which was effective against bFGF-induced tube formation.
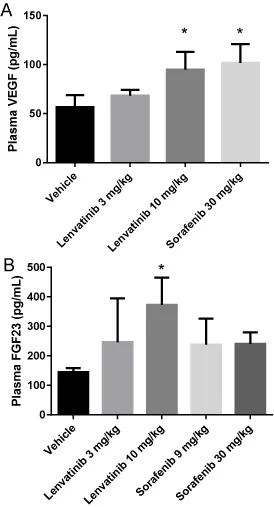
Up-regulation of plasma VEGF and FGF23 levels by the administration of lenvatinib to mice
To evaluate the inhibitory activity of lenvatinib and sorafenib against VEGFR and FGFR in vivo, we measured the plasma levels of VEGF and FGF23, both of which are considered to be response biomarkers for VEGFR and FGFR blockade, respectively. Patients treated with VEGFR inhibitors such as sorafenib, sunitinib, and axitinib demonstrate increased plasma VEGF levels [26-28]. In addition, VEGFR-targeting agents induced increases in plasma VEGF concentrations in a preclinical mouse model [15,16], suggesting that the up-regulation of VEGF in plasma is a class effect for VEGFR signaling inhibition. Although the lower dose of lenvatinib (3 mg/kg) demonstrated a nonsignificant tendency (P>0.1) to elevate plasma VEGF, lenvatinib at 10 mg/kg induced a significant elevation of VEGF levels. In comparison, sorafenib at 30 mg/kg significantly enhanced plasma VEGF level in mice (Figure 3A). These data indicate that both lenvatinib and sorafenib suppress VEGFR activity in vivo.
FGF23 is known as a biomarker of FGFR pathway inhibition. Inhibition of FGFR signaling induces the secretion of vitamin D from the kidney, and then the vitamin D up-regulates transcriptional expression of FGF23 in bone cells [17]. We therefore measured the plasma FGF23 levels of mice to assess whether lenvatinib or sorafenib blocks FGFR signaling in vivo. The administration of lenvatinib at 10 mg/kg significantly elevated plasma FGF23 levels, whereas sorafenib at 30 mg/kg did not (Figure 3B). These results support the conclusion that lenvatinib but not sorafenib inhibits FGFR activity in an in vivo mouse model.
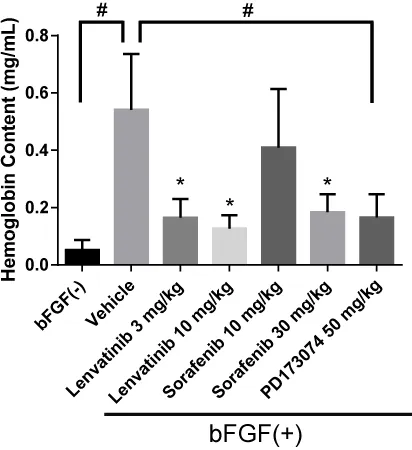
Inhibitory activities of lenvatinib and sorafenib in bFGF-driven Matrigel angiogenesis model in vivo
Finally, to elucidate the inhibitory activity of lenvatinib against bFGF-driven angiogenesis in vivo, we used a Matrigel plug assay. Because the hemoglobin content in the gel matrix reportedly correlated with the number of blood vessels formed, the concentration of hemoglobin was measured as an index of angiogenesis [29]. Lenvatinib suppressed bFGF-driven angiogenesis at doses of both 3 and 10 mg/kg (Figure 4); PD173074, a selective FGFR inhibitor [30], showed anti-angiogenic effects as well (Figure 4). Sorafenib disrupted bFGF-driven angiogenesis, albeit only at the higher dose of 30 mg/kg, probably because of its VEGFR-inhibitory activity, given that bFGF itself reportedly up-regulates VEGF expression [31]. Therefore, the VEGF secreted in response to bFGF stimulation may contribute to angiogenesis in our Matrigel assay. In contrast to sorafenib, lenvatinib disrupted bFGF-driven angiogenesis even at the lower dose (3 mg/kg), suggesting that lenvatinib suppresses angiogenesis by simultaneous targeting of both VEGFR and FGFR activities.
Discussion
In the clinical setting, complete tumor responses to VEGF-targeted drugs are uncommon, and most patients develop resistance to these agents through the activation of alternative pathways, such as those involving FGF, angiopoietin, and ephrin [32]. In the RIP-Tag2 mouse model of islet cell carcinogenesis, an anti-VEGFR2 antibody (DC101) demonstrated marked antitumor activity after short-term treatment (10 days) [33]. In contrast, long-term treatment (4 weeks) with the anti-VEGFR2 antibody was associated with tumor regrowth and no significant difference in tumor burden between control and treatment groups because of up-regulation of pro-angiogenic factors including bFGF in the tumor tissue. Indeed, the combination of anti-VEGFR2 antibody and FGF-trap treatments led to significant decreases in tumor burden and vessel density compared with either treatment alone [33]. Furthermore, blockade of VEGF–VEGFR signaling by using agents such as bevacizumab elevated bFGF levels in patient’s plasma [14]. Thus, jointly inhibiting both the VEGFR and FGFR signaling pathways might provide significant antitumor effects for cancer patients.
In the current study, lenvatinib suppressed the tube formation induced by bFGF and by bFGF plus VEGF with IC50 values of approximately 10 nmol/L (Figure 2); in comparison, lenvatinib inhibited VEGF with an IC50 of approximately 3 nmol/L in a previous report [19]. These results show that lenvatinib harbors anti-angiogenic activity against both VEGF and bFGF at close dosage ranges. Therefore, lenvatinib might abrogate the resistance mechanisms associated with conventional VEGF-targeted drugs.
In an in vivo angiogenesis model, bFGF reportedly triggered the secretion of VEGFs from fibroblasts, and the released VEGFs were considered to induce angiogenesis in mice [34]. Therefore, not only bFGF but also VEGF may act as a pro-angiogenic factor in our Matrigel plug assay. In this context, sorafenib (at increased doses) might inhibit bFGF-driven angiogenesis through its targeting of VEGFR (Figure 4).
Lenvatinib suppressed the in vitro proliferation of cancer cells in which FGFR2 or FGFR3 is mutated or amplified, such as KMS-11, KATO III, SNU-16, and HSC-39 (data not shown). Moreover, we elucidated that lenvatinib suppressed tumor growth of the thyroid cancer cell line RO82-W1, which overexpresses FGFR1, and K1, in a mouse xenograft model (Figure S2) [20]. These results suggest that lenvatinib might provide not only anti-angiogenic effects but also cause direct antitumor effects through the inhibition of the FGFR signaling pathway in cancer cells.
Collectively, our current results suggest that the anti-angiogenic activity of lenvatinib is more potent than that of sorafenib and might reflect dual inhibition of both the VEGFR and FGFR signaling pathways. Lenvatinib was recently approved for use against advanced or differentiated thyroid cancer refractory to radioactive iodine, in which it achieved an overall response rate of 64.8% and a complete response rate of 1.5% [24]. In contrast, the overall and complete response rates of sorafenib were 12.2% and 0%, respectively [9]. We acknowledge that the lenvatinib and sorafenib trials were conducted independently and that comparisons of the resulting data should be interpreted with caution. Nevertheless our results suggest that lenvatinib’s inhibitory effects on both FGFR and VEGFR might offer additional therapeutic benefit to cancer patients. Preclinical and clinical studies that further investigate the dual inhibition of VEGFR and FGFR by lenvatinib are warranted.
We thank Naoko Tsukahara and Kyoko Yoshizawa for establishment of tube length calculating system in in vitro angiogenesis assay./p>
- Potente M, Gerhardt H, Carmeliet P (2011) Basic and therapeutic aspects of angiogenesis. Cell 146: 873-887.
- Ellis LM, Hicklin DJ (2008) VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 8: 579-591.
- Bernatchez PN, Soker S, Sirois MG (1999) Vascular endothelial growth factor effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis is Flk-1-dependent. J Biol Chem 274: 31047–31054.
- Murakami M, Nguyen LT, Zhuang ZW, Moodie KL, Carmeliet P, et al. (2008) The FGF system has a key role in regulating vascular integrity. J Clin Invest 118: 3355-3366.
- Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K (2011) VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol Med 17: 347-362.
- Dixelius J, Makinen T, Wirzenius M, Karkkainen MJ, Wernstedt C, et al. (2003) Ligand-induced Vascular Endothelial Growth Factor Receptor-3 (VEGFR-3) heterodimerization with VEGFR-2 in primary lymphatic endothelial cells regulates tyrosine phosphorylation sites. J Biol Chem 278: 40973-40979.
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L (2006) VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol 7: 359-371.
- Welti J, Loges S, Dmmeler S, Carmeliet P (2013) Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest 123: 3190-3200.
- Brose MS, Nutting CM, Jarzab B, Elisei R Siena S, et al. (2014) Sorafenib in locally advanced or metastatic, radioactive iodine-refractory, differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. The Lancet 384: 319-328.
- Michael JC, Claesson-Welsh L (2001) FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends in Pharmacological Sciences 22: 201-207.
- Sonpavde G, Willey CD, Sudarshan S (2014) Fibroblast growth factor receptors as therapeutic targets in clear-cell renal cell carcinoma. Expert Opin Investig Drugs 23: 305-315.
- Cheng AL, Shen YC, Zhu AX (2011) Targeting fibroblast growth factor receptor signaling in hepatocellular carcinoma. Oncology 81: 372-380.
- Amelia C, Petra W, Maria-Antonietta I, Matt C, Gerhard C (2000) Fibroblast growth factors are required for efficient tumor angiogenesis. Cancer Research 60: 7163-7169.
- Murukesh N, Dive C, Jayson GC (2010) Biomarkers of angiogenesis and their role in the development of VEGF inhibitors. Br J Cancer 102: 8-18.
- Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang PY, et al. (2010) Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res 16: 3420-3430.
- Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, and Kerbel RS (2007) Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci USA 104: 17069-17074.
- Wohrle S, Bonny O, Beluch N, Gaulis S, Stamm C, et al. (2011) FGF receptors control vitamin D and phosphate homeostasis by mediating renal FGF-23 signaling and regulating FGF-23 expression in bone. J Bone Miner Res 26: 2486-2497.
- Okamoto K, Ikemori-Kawada M, Jestel A, Konig K, Funahashi Y, et al. (2015) Distinct binding mode of multikinase inhibitor lenvatinib revealed by biochemical characterization. ACS Med Chem Lett 6: 89-94.
- Yuji Y, Junji M., Tomohiro M, Hiroshi O, Kazuki M, et al. (2014) Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vascular Cell 6: 18.
- Tohyama O, Matsui J, Kodama K, Hata-Sugi N, Kimura T, et al. (2014) Antitumor activity of lenvatinib (E7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res 2014: 638747.
- Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, et al. (2008) Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of Vascular Endothelial Growth Factor-Receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res 14: 5459-5465.
- Matsui J, Yamamoto Y, Funahashi Y, Tsuruoka A, Watanabe T, et al. (2008) E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer 122: 664-671.
- Okamoto K, Kodama K, Takase K, Hata-Sugi N, Yamamoto Y, et al. (2013) Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett 340: 97-103.
- Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, et al. (2015) Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372: 621-630.
- Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, et al. (2015) Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. The Lancet Oncology 16: 1473-1482.
- Tsuchiya K, Asahina Y, Matsuda S, Muraoka M, Nakata T, et al. (2014) Changes in plasma vascular endothelial growth factor at 8 weeks after sorafenib administration as predictors of survival for advanced hepatocellular carcinoma. Cancer 120: 229-237.
- Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, et al. (2008) Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 26: 1810-1816.
- Bruce JY, Scully PC, Carmichael LL, Eickhoff JC, Perlman SB et al. (2015) Pharmacodynamic study of axitinib in patients with advanced malignancies assessed with 18F-3'deoxy-3'fluoro-L-thymidine positron emission tomography/computed tomography. Cancer Chemother Pharmacol 76: 187-195.
- Passaniti A, Taylor R, Pili R, Guo Y, Long PV, et al. (1992) A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Laboratory Investigation 67: 519-528.
- Moosa M, Scott F, James MH, Mel CS, Robert LP, et al. (1998) Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. The EMBO Journal 17: 5896-5904.
- Graziano S, Sundeep P, Christine JR, Anna G, Giuseppe P, et al. (1998) Fibroblast Growth Factor-2 (FGF-2) induces Vascular Endothelial Growth Factor (VEGF) expression in the endothelial cells of forming capillaries an autocrine mechanism contributing to angiogenesis. The Journal of Cell Biology 141: 1659-1673.
- Bergers G and Hanahan D (2008) Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 8: 592-603.
- Casanovas O, Hicklin DJ, Bergers G, Hanahan D (2005) Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 8: 299-309.
- Kevin PC, Kristin A, Shu-Ching S, Lawrence FB, Andrew M, et al. (2001) Fibroblast growth factor 2 activation of stromal cell vascular endothelial growth factor expression and angiogenesis. Laboratory Investigation 81: 61-75.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
