Annals of Bone Marrow Research
Using recombinant human G-CSF to treat chemotherapy-induced neutropenia over 3 decades: What is next?
Jason Xu1,2, Jonathan Sussman1,2, Jessica Xu3, Xing Zhao4 and Xiao Qiang Yan4*
2Medical Scientist Training Program, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
3College of Arts and Sciences, University of Pennsylvania, Philadelphia, PA, USA
4iTabMed (Shanghai) Ltd. Pudong, Shanghai, China
Cite this as
Xu J, Sussman J, Xu J, Zhao X, Yan XQ (2022) Using recombinant human G-CSF to treat chemotherapy-induced neutropenia over 3 decades: What is next? Ann Bone Marrow Res 8(1): 001-004. DOI: 10.17352/abmr.000010Copyright Licence
© 2023 Xu J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Chemotherapy-Induced Neutropenia (CIN) is a potentially fatal side effect of cancer treatment, affecting > 50% of cancer patients treated with chemotherapy. Clinical use of recombinant human granulocyte colony-stimulating factor (rhG-CSF) has allowed for primary and secondary prophylaxis of CIN and its sequela (i.e., febrile neutropenia, fatal infection) during myelosuppressive chemotherapy. Here, we review the translation and properties of first, second, and third-generation rhG-CSF molecules, including filgrastim (Neupogen, FDA approved in 1991) and biosimilars, pegfilgrastim (Neulasta, FDA approved in 2002) and biosimilars, and F-627 (Ryzneuta, NMPA approved in 2023), a novel long-acting rhG-CSF agent developed this past decade. Even with the development of increasingly personalized and targeted cancer therapy, chemotherapy, and stem cell transplantation remains a backbone for the majority of patients with advanced cancers, especially in the hematopoietic system. As such, more than 20 million cancer patients have been treated with rhG-CSF drugs since the first approval of filgrastim. In the next decade, we envision third-generation rhG-CSF products such as Ryzneuta lowering costs to patients and healthcare providers, expanding access to this essential medication for cancer patients worldwide, particularly for patients who require more aggressive chemotherapy treatment.
Introduction
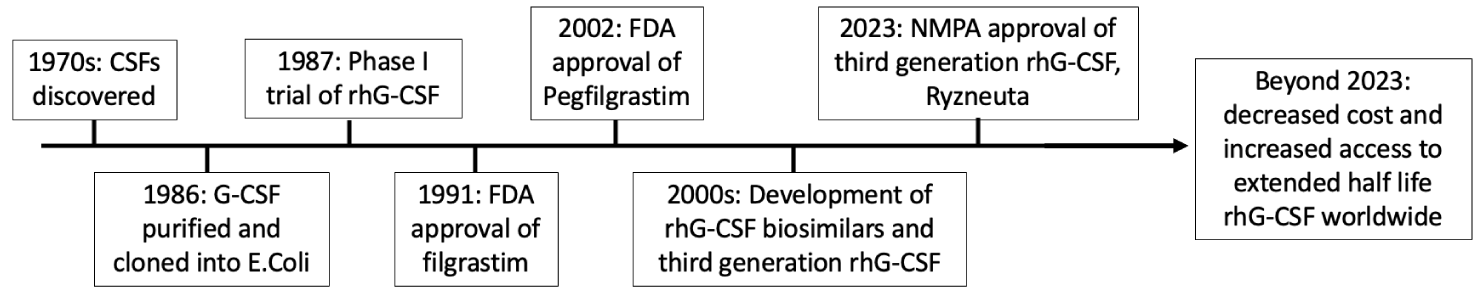
Chemotherapy-Induced Neutropenia (CIN) is a common and potentially fatal side effect of cancer treatment, affecting >50% of cancer patients treated with chemotherapy. Patients with prolonged neutropenia are at risk for severe infection, with the degree of granulocyte depletion directly correlating with infection risk and severity [1]. Clinical use of recombinant human granulocyte colony-stimulating factor (rhG-CSF) in the last three decades has allowed for primary and secondary prophylaxis of CIN and its sequela (i.e., febrile neutropenia, fatal infection) during myelosuppressive chemotherapy (Figure 1). Below, we provide a brief review of the discovery and translation of G-CSF biology into clinical practice with emphasis on first-generation, second-generation, and emerging next-generation rhG-CSF-based therapeutics.
Review: Recombinant human G-CSF to treat chemotherapy-induced neutropenia
Colony-Stimulating Factors (CSFs) were first discovered as molecules able to promote the growth of myeloid cells and myeloid leukemia in vitro [2,3]. In the 1970s, four distinct CSFs—GM-CSF, G-CSF, M-CSF, and IL-3 – were identified and named after the type of differentiation they induced in bone marrow progenitor’s ex vivo [2] (Figure 1, “1970s:”). Of these, Granulocyte Colony Stimulating Factor (G-CSF) was found to stimulate neutrophil colony formation [4] and inversely correlate with neutrophil levels in vivo, with upregulation of several magnitudes during acute infection [5,6].
Human G-CSF was first purified and subsequently cloned from human bladder carcinoma cell line 5637 by Karl Welte’s group in 1986 [7] (Figure 1, “1986:”). Human G-CSF was purified from 5637 cells via polyacrylamide gel at > 95% purity and sequenced via high-performance liquid chromatography, allowing for the construction of oligonucleotide probes to capture candidate mRNAs. Cloning into cDNA expression vectors subsequently allowed for efficient and specific expression of recombinant human G-CSF in E. coli [7]. Notably, the purified recombinant protein induced granulocyte differentiation of immature myeloid and leukemic cell lines into terminally differentiated cells, including band forms, segmented neutrophils, and monocytes/macrophages.
The first generation of rhG-CSF entering clinical development was filgrastim (brand name: Neupogen), a short-half-life rhG-CSF produced in E. coli. The first rhG-CSF clinical studies on 24 advanced cancer patients demonstrated rapid production of functional neutrophils upon continuous infusion [8] (Figure 1, “1987:”). Notably, even in this limited cohort, rhG-CSF was seen to reduce the duration of absolute neutropenia and subsequent infection by 80% [8]. In 1991, the US FDA approved the first rhG-CSF product, filgrastim for clinical use based on the results of a phase III trial enrolling 210 patients with small cell lung cancer [9-11,19] (Figure 1, “1991:”). Compared to placebo, daily subcutaneous administration of filgrastim (brand name: Neupogen) during cyclophosphamide/doxorubicin/etoposide chemotherapy led to a significant reduction in the duration of severe neutropenia (6 days vs. 2 days), febrile neutropenia (76% vs. 40%, p < 0.001), IV antibiotic use (60% vs. 38%), and hospitalization rate (69% vs. 52%). Due to its half-life of 3.5 hours, filgrastim requires daily administration after chemotherapy.
The second generation of rhG-CSF drugs, including pegylated filgrastim (Pegfilgrastim), were developed as extended half-life agents to enable once-per-cycle, rather than daily, dosing. Pegfilgrastim is produced through covalent attachment of PEG to the N-terminal amine group of rhG-CSF so that the molecule becomes too large for renal clearance [12], thereby increasing the serum half-life by over ten-fold (~3.5 hours vs. ~42 hours). In 2002, the FDA approved Pegfilgrastim based on its non-inferiority to filgrastim to treat CIN in two international randomized trials enrolling a total of 467 breast cancer patients [13] (Figure 1, “2002:”). Compared to filgrastim, Pegfilgrastim demonstrated non-inferiority in duration of severe neutropenia and rates of febrile neutropenia with once per chemotherapy cycle compared to daily dosing of filgrastim [13]. Currently, there are over 20 biosimilars of Filgrastim and Pegfilgrastim (9 US FDA approved, Table 1) available in different countries for treatment of CIN and other neutropenic conditions (Table 2) [14]. Pegylated rhG-CSF is administered to cancer patients after chemotherapy to manage CIN. However, due to the high cost of pegylated rhG-CSF, short-acting rhG-CSF is still widely used in less developed countries. In addition, pegylation of rhG-CSF reduces the receptor binding affinity to G-CSF receptors due to increased hydrophobia of the molecule [15]. Thus, in high-dose chemotherapy settings severe neutropenia and febrile neutropenia (FN) can still occur. Other technologies to extend the half-life of G-CSF, such as fusion of albumin to G-CSF did not reach clinical development endpoints [16,17].
Discussion: What’s next for the management of CIN?
Even with the development of increasingly personalized and targeted cancer therapy, chemotherapy, and stem cell transplantation remains a backbone for the majority of patients with advanced tumors, especially in the hematopoietic system. The primary prevention of neutropenic complications in these patients has made rhG-CSF a WHO essential medicine [20] and part of cancer care guidelines for most international cancer working groups [21]. Over the last decade, third-generation rhG-CSF proteins, such as F-627 (brand name: Ryzneuta), were developed with the goal of preserving the biological activity of native rhG-CSF while retaining the clinical advantages of extended half-life rhG-CSF (Figure 1, “Beyond 2023:”). Unlike Pegfilgrastim and long-acting biosimilars, F-627 is produced in CHO cells and assembles into a rhG-CSF dimer linked by a human IgG2 Fc backbone. This unique design allows native affinity to G-CSF receptor binding with more potent receptor activation due to induced dimerization of the G-CSF receptor. In addition, due to its large molecular weight and capacity for Fc recycling, F-627 retains the extended half-life of rhG-CSFs. In a CIN monkey model, F-627 demonstrated more rapid neutrophil recovery with a shortened duration of neutropenia compared with Pegfilgrastim at an equal molar dosage [27]. Phase II and Phase III studies of F-627 were conducted head-to-head with filgrastim and pegfilgrastim. In two phase III non-inferiority studies, F-627 was found to be non-inferior to filgrastim and pegfilgrastim at all primary endpoints. In addition, when compared to filgrastim, F-627 demonstrated lower incidence and shorter duration of grade 3/4 neutropenia, higher ANC nadir, and was associated with decreased incidence of chemotherapy dose reduction [18]. With its efficient manufacturing process that mitigates the complexity and risks of PEGylation, F-627 has promise to bridge long-acting, once-per-cycle rhG-CSF treatment to more patients worldwide. In intensive chemotherapy settings, F-627 may have advantages in shortening the duration of severe neutropenia or reducing the incidence of FN, which remains to be demonstrated in patients. BLA (biological license application) filings to FDA and EMA (European Medicines Agency) have been completed. The approval of Ryzneuta by the FDA and EM is expected to be in the near future.
Conclusions & Future perspectives
As of this decade, more than 20 million cancer patients have been treated with rhG-CSF drugs. The first rhG-CSF agent, filgrastim, was purified and recombinantly expressed in 1986 by Karl Welte’s group. Within pivotal trials, daily administration of filgrastim significantly reduced the duration of severe neutropenia and febrile neutropenia leading to FDA approval in 1991. Subsequently, PEGylated filgrastim (pegfilgrastim) was developed as an extended half-life alternative to filgrastim, ultimately receiving FDA approval in 2002 with similar efficacy demonstrated in non-inferiority trials versus filgrastim. Novel third-generation rhG-CSF agents such as Ryzneuta (NMPA approved in 2023) have emerged, combining the biological advantages of first and second-generation rhG-CSF products with the manufacturing advantages of CHO cell production (Figure 1). In the coming decade, we envision third-generation rhG-CSFs decreasing costs and increasing access to essential rhG-CSF therapies for cancer patients worldwide.
- Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966 Feb;64(2):328-40. doi: 10.7326/0003-4819-64-2-328. PMID: 5216294.
- Nicola NA, Metcalf D, Johnson GR, Burgess AW. Separation of functionally distinct human granulocyte-macrophage colony-stimulating factors. Blood. 1979 Sep;54(3):614-27. PMID: 313822.
- Panopoulos AD, Watowich SS. Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and 'emergency' hematopoiesis. Cytokine. 2008 Jun;42(3):277-88. doi: 10.1016/j.cyto.2008.03.002. Epub 2008 Apr 8. PMID: 18400509; PMCID: PMC2852428.
- Nicola NA, Metcalf D, Matsumoto M, Johnson GR. Purification of a factor inducing differentiation in murine myelomonocytic leukemia cells. Identification as granulocyte colony-stimulating factor. J Biol Chem. 1983 Jul 25;258(14):9017-23. PMID: 6190815.
- Dale DC. The discovery, development and clinical applications of granulocyte colony-stimulating factor. Trans Am Clin Climatol Assoc. 1998;109:27-36; discussion 36-8. PMID: 9601125; PMCID: PMC2194342.
- Metcalf D. The role of the colony-stimulating factors in resistance to acute infections. Immunol Cell Biol. 1987 Feb;65 ( Pt 1):35-43. doi: 10.1038/icb.1987.4. PMID: 3301635.
- Souza LM, Boone TC, Gabrilove J, Lai PH, Zsebo KM, Murdock DC, Chazin VR, Bruszewski J, Lu H, Chen KK, Barendt J, Platzer E, Moore MAS, Mertelsmann R, Welte K. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986 Apr 4;232(4746):61-5. doi: 10.1126/science.2420009. PMID: 2420009.
- Bronchud MH, Scarffe JH, Thatcher N, Crowther D, Souza LM, Alton NK, Testa NG, Dexter TM. Phase I/II study of recombinant human granulocyte colony-stimulating factor in patients receiving intensive chemotherapy for small cell lung cancer. Br J Cancer. 1987 Dec;56(6):809-13. doi: 10.1038/bjc.1987.295. PMID: 2829955; PMCID: PMC2002403.
- Welte K, Gabrilove J, Bronchud MH, Platzer E, Morstyn G. Filgrastim (r-metHuG-CSF): the first 10 years. Blood. 1996 Sep 15;88(6):1907-29. PMID: 8822908.
- Dale DC, Bonilla MA, Davis MW, Nakanishi AM, Hammond WP, Kurtzberg J, Wang W, Jakubowski A, Winton E, Lalezari P, et al. A randomized controlled phase III trial of recombinant human granulocyte colony-stimulating factor (filgrastim) for treatment of severe chronic neutropenia. Blood. 1993 May 15;81(10):2496-502. PMID: 8490166; PMCID: PMC4120868.
- Aghedo BO, Gupta V. Filgrastim. 2023 Jul 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. PMID: 32644708.
- Molineux G. The design and development of pegfilgrastim (PEG-rmetHuG-CSF, Neulasta). Curr Pharm Des. 2004;10(11):1235-44. doi: 10.2174/1381612043452613. PMID: 15078138.
- Drug Approval Package: Neulasta (Pegfilgrastim) NDA #125031. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/125031_0000_NeulastaTOC.cfm.
- Welte K. G-CSF: filgrastim, lenograstim and biosimilars. Expert Opin Biol Ther. 2014 Jul;14(7):983-93. doi: 10.1517/14712598.2014.905537. Epub 2014 Apr 7. PMID: 24707817.
- Scholz M, Ackermann M, Engel C, Emmrich F, Loeffler M, Kamprad M. A pharmacokinetic model of filgrastim and pegfilgrastim application in normal mice and those with cyclophosphamide-induced granulocytopaenia. Cell Prolif. 2009 Dec;42(6):813-22. doi: 10.1111/j.1365-2184.2009.00638.x. Epub 2009 Aug 17. PMID: 19689472; PMCID: PMC6496189.
- Strohl WR. Fusion Proteins for Half-Life Extension of Biologics as a Strategy to Make Biobetters. BioDrugs. 2015 Aug;29(4):215-39. doi: 10.1007/s40259-015-0133-6. PMID: 26177629; PMCID: PMC4562006.
- May 31 & 2011 08:22am. Teva Announces Data on Granulocyte Colony Stimulating Factor Compounds for the Prevention of Chemotherapy-Induced Neutrope. Fierce Biotech. https://www.fiercebiotech.com/biotech/teva-announces-data-on-granulocyte-colony-stimulating-factor-compounds-for-prevention-of (2011).
- Efbemalenograstim alfa, a long-acting granulocyte colony-stimulating factor, a novel dimeric G-CSF Fc fusion protein for reducing the risk of febrile neutropenia following chemotherapy. https://probiologists.com/Article/Efbemalenograstim-alfa,-a-long-acting-granulocyte-colony-stimulating-factor,-a-novel-dimeric-G-CSF-Fc-fusion-protein-for-reducing-the-risk-of-febrile-neutropenia-following-chemotherapy.
- WEHI History: 1991 CSFs Approved for Clinic after 25-Year Journey. WEHI https://www.wehi.edu.au/about/history/csfs-approved/.
- WHO Model List of Essential Medicines - 23rd list, 2023. https://www.who.int/publications-detail-redirect/WHO-MHP-HPS-EML-2023.02.
- Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, Goldberg JM, Khatcheressian JL, Leighl NB, Perkins CL, Somlo G, Wade JL, Wozniak AJ, Armitage JO; American Society of Clinical Oncology. Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2015 Oct 1;33(28):3199-212. doi: 10.1200/JCO.2015.62.3488. Epub 2015 Jul 13. PMID: 26169616.
- Research C. for DE and. Biosimilar Product Information. FDA (2023).
- Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, Kris M, Grous J, Picozzi V, Rausch G, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991 Jul 18;325(3):164-70. doi: 10.1056/NEJM199107183250305. PMID: 1711156.
- Heil G, Hoelzer D, Sanz MA, Lechner K, Liu Yin JA, Papa G, Noens L, Szer J, Ganser A, O'Brien C, Matcham J, Barge A. A randomized, double-blind, placebo-controlled, phase III study of filgrastim in remission induction and consolidation therapy for adults with de novo acute myeloid leukemia. The International Acute Myeloid Leukemia Study Group. Blood. 1997 Dec 15;90(12):4710-8. PMID: 9389686.
- Sheridan WP, Morstyn G, Wolf M, Dodds A, Lusk J, Maher D, Layton JE, Green MD, Souza L, Fox RM. Granulocyte colony-stimulating factor and neutrophil recovery after high-dose chemotherapy and autologous bone marrow transplantation. Lancet. 1989 Oct 14;2(8668):891-5. doi: 10.1016/s0140-6736(89)91552-3. PMID: 2477656.
- Schmitz N, Linch DC, Dreger P, Goldstone AH, Boogaerts MA, Ferrant A, Demuynck HM, Link H, Zander A, Barge A. Randomised trial of filgrastim-mobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. Lancet. 1996 Feb 10;347(8998):353-7. doi: 10.1016/s0140-6736(96)90536-x. Erratum in: Lancet 1996 Mar 30;347(9005):914. PMID: 8598700.
- MacVittie TJ, Bennett AW, Farese AM, Taylor-Howell C, Smith CP, Gibbs AM, Prado K, Jackson W 3rd. The Effect of Radiation Dose and Variation in Neupogen® Initiation Schedule on the Mitigation of Myelosuppression during the Concomitant GI-ARS and H-ARS in a Nonhuman Primate Model of High-dose Exposure with Marrow Sparing. Health Phys. 2015 Nov;109(5):427-39. doi: 10.1097/HP.0000000000000350. PMID: 26425903; PMCID: PMC9442798.
- Hankey KG, Farese AM, Blaauw EC, Gibbs AM, Smith CP, Katz BP, Tong Y, Prado KL, MacVittie TJ. Pegfilgrastim Improves Survival of Lethally Irradiated Nonhuman Primates. Radiat Res. 2015 Jun;183(6):643-55. doi: 10.1667/RR13940.1. Epub 2015 Jun 2. PMID: 26035709.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
