Annals of Bone Marrow Research
T-ALL with TEL/AML1 Translocation, Aberrant Expression of CD19 and 33: Case Report and Literature Review
Kave Jasseb1, Maria Kavianpour1, Javad Mohammadi Asl2, Zahra Shahpouri Arani1, Vahid Fallah Azad3, Mansoureh Haghighi3 and Najmaldin Saki1*
2Department of Medical Genetics, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Iran
3Research Department, Mahak’s Pediatric Cancer Treatment and Research Center, Tehran, Iran
Cite this as
Jasseb K, Kavianpour M, Asl JM, Arani ZS, Azad VF, et al. (2016) T-ALL with TEL/AML1 Translocation, Aberrant Expression of CD19 and 33: Case Report and Literature Review. Ann Bone Marrow Res 1(1): 001-004, DOI: 10.17352/abmr.000001We herewith introduce a 9-year-old boy presenting with leukocytosis, anemia and high lymphoblast count who had a pale complexion as well as weight loss. His cytogenetic analysis revealed aberrant chromosomal rearrangements in different clonal populations harboring 46XY karyotype with t (12; 21) (p12; q22), which was confirmed by DNA sequencing. Flowcytometry assay detected aberrant B lymphocyte and myeloid CD markers such as CD19 (22.0%) and CD33 (20.5%), respectively. To our knowledge, this is the first case of a patient initially diagnosed as TEL/AML1 transcript positive T-ALL expressing CD19 and CD33 markers. The present article also highlights the need for molecular gene rearrangement studies to determine the precise lineage of ambiguous ALL clones.
Abbreviations
M: Male; CD: Cluster of Differentiation; TDT: Terminal Deoxynucleotidyl Transferase; MPO: Myeloperoxidase; IgM: Immunoglobulin M; HLA: Human Leukocyte Antigen
Introduction
T-Acute lymphoblastic leukemia (T-ALL) is one of the most important hematologic malignancies occurring due to clonal proliferation of T lymphocytes in approximately 15-25% of ALL patients [1]. In comparison with B-ALL patients, those suffering from T-ALL are at risk of increased leukocyte count (WBC>20×109/L), organomegaly, enlarged mediastinal mass and central nervous system (CNS) involvement [2]. Immunophenotype studies have enabled the differentiation between T- and B-cells. Cluster of differentiation (CD) molecules 79a and CD22 as well as cCD3 and TCR are specific for B-cells and T-cells, respectively. Myeloid markers such as CD13, CD33 and myeloperoxidase (MPO) may be observed in different ALL types, and MPO expression can be a marker of ambiguous lineage leukemia, for example in T or B ALL with aberrant expression of myeloid lineage markers [3].
Several chromosomal abnormalities may be detected in this type of leukemia, including t (1; 19) (q23; p13), t (11; 19) (q23; p13.3), t(4; 11) (q21; q23), t(9; 22) (q34; q11), hyper- and hypo-diploidy as well as t(12; 21) (p12; q22) [4]. t-(12; 21) (p12; q22) is caused by fusion of part of Translocation-Ets-Leukemia (TEL) (12p12) gene within acute myeloid leukemia 1 (AML1) (21q22) gene. This translocation has been reported to be highly frequent in 1-12 year old children but it is rarely observed in childhood AML and adult ALL [2,5].
Immunophenotype study of this translocation revealed the presence of CD10, CD19 and CD22, precursor B-cell phenotype as well as other markers of this cell line such as HLA-DR, CD10, CD13, CD19, CD24, CD33, CD34, CD40, CD45 and CDw65 but the absence of CD9, CD20 and CD86 [6-8]. In general, t(12; 21) (p12; q22) is observed in common ALL, pre-B-ALL (BCP-ALL) and rarely in pro-B-ALL, and is raised as the most common gene rearrangement in childhood BCP-ALL [9,10]. In the study of Ma et al, one of the patients had T-ALL, which has been the only report of t(12; 21) translocation in T-ALL [11].
The presence of t(12; 21) translocation usually involves a favorable prognosis and response to treatment since the majority of TEL/AML1 positive patients show non-invasive clinical symptoms, non-hyperdiploid DNA content and younger than 10 years of age [12-14]. Favorable prognosis resulting from this fusion can be demonstrated with its low incidence in recurrent cases (8.9% and 10%) in addition to high percentage of event-free survival (EFS) (approximately 89-100%) [13,15,16]. Detection of TEL/AML1 gene fusion is considered an important marker for prognosis of response to treatment. In this study, we have investigated a patient with diagnosis of T-ALL having t(12;21) (p12; q22) translocation, which has not been reported except for one case.
Case Presentation
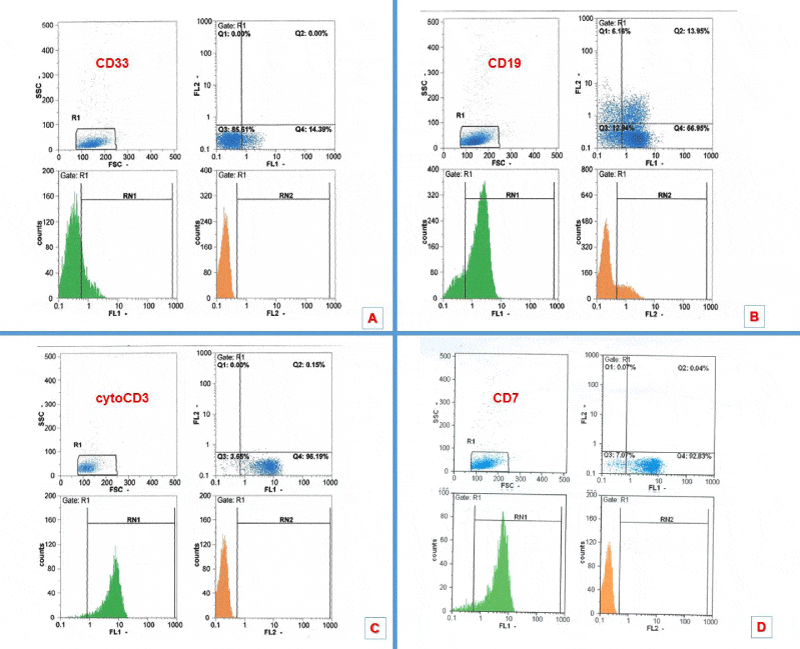
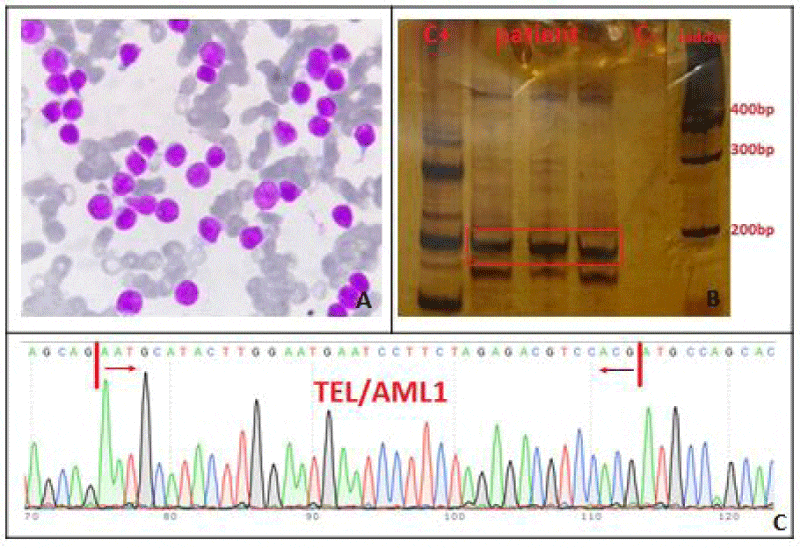
A 9-year-old boy with a pale complexion and weight loss (BMI<20.5 upon admission) for several weeks was referred to Shafa Hospital in Ahwaz. Peripheral blood (PB) sample was taken and bone marrow (BM) aspiration was done for further investigation. Initial ultrasound of patient’s abdomen showed normal liver size but a spleen size of 159 mm, which was larger than normal. Complete blood count (CBC) showed leukocytosis and anemia (WBC: 172.3 ×109/L, Hemoglobin (Hb): 7.3 g/dL, Red blood cell (RBC): 2.68 ×1012/L) but other hematologic and biochemical tests were normal. Wright-Giemsa staining of BM smear indicated a high count of lymphoblasts (Figure 1). EDTA-anticoagulated BM sample was investigated to determine the involved cell line and specific expression of CD markers. The results indicated the expression of markers such as CD7 (94.0%), CD19 (22.0%), CD33 (20.5%), cCD3 (95.6%), TdT (29.4%), MPO (17.2%), CD38 (99.4%), CD2 (83.8%), CD5 (68%), CD19 (22%), CD34 (37.5%) and cCD79a (15.2%). The BM sample showed t(12:21), and the presence of TEL/AML1 fusion was confirmed by molecular methods and sequencing. After purification RNA and cDNA production, the two steps of nested-polymerase chain reaction (Nested-PCR) were performed and the primer sequences are listed in Table.1. Then, PCR products were electrophoresed on acrylamide gel. Amplification of TEL/AML1 transcript was used as the positive and negative control. PCR product of TEL/AML1 transcript was sequenced using ABI PRISM 3730 DNA Analyzer (Applied Biosystems, Foster City, CA) (Figure 2).
The patient received Vincristine (VCR), L-Asparaginase (L-ASP), Prednisolone and Daunorubicin as induction and was discharged with improved health status. For complete remission he received Prednisolone, 6-Mercaptopurin, Vincristine and Methotrexate.
Discussion
Translocation (12; 21) (p12; q22) is one of the most important genetic lesions occurring in ALL. This fusion is caused by integration of two critical genes involved both in hematopoiesis and leukemogenesis. Cytogenetic and molecular studies such as fluorescent in situ hybridization (FISH) and real-time PCR (RT-PCR) have enabled the detection of this translocation and its subsequent fusion [13,15]. After detection of this translocation by Shurtleff et al., TEL/AML1 fusion was introduced as an ‘’excellent prognosis’’ marker, although 5-year event free survival in patients harboring this fusion was not significantly different from other patients who were negative for it [6]. In general, the presence of this translocation places the patients in a separate group with low risk for recurrence and better response to treatment. According to different studies, it is believed that the presence of TEL/AML1 fusion is indicative of B-ALL. In addition, presence of this fusion is associated with lower leukocyte count and lower age (1-10 years), which are considered as favorable prognostic factors [17].
Study of immunophenotype is an important biological parameter in patient’s survival and prediction of response to treatment. Aberrant phenotype occurs with the development of lymphoid and myeloid markers in myoblasts or lymphoblasts, and has an overall frequency of 80% in both ALL and AML [18]. Specific T-lymphoid markers include CD1a, CD3, CD4, CD5, CD7 and CD8 but B-lymphoid antigens are CD10, CD19, CD22, and cytoplasmic CD79a. MPO, CD13 and CD33 are also common myeloid markers. CD34, HLA-DR, and TdT are used to investigate the precursor cells (Table 2). The world health organization defines Pre-T ALL as a malignancy often occurring in TdT-positive males, which is sometimes associated with the expression of CD10, CD79a and cCD3 markers. Myeloid markers (CD13 and CD33) are “often present” but CD117 is rarely observed [19]. In the study of Suggs et al, the expression of CD13 and CD33 was reported in 24% of pre-T ALL cases, who were male patients aged 13-25 years [20]. The study of Yao et al, dealt with a patient suffering from T-cell lymphoma with aberrant expression of CD79a and CD20. The patient had both B- and T-cell markers and was deceased six months after initial diagnosis due to poor prognosis. This study also showed that the presence of a marker is not specific to a certain disease type and a panel of antibodies should be used for further investigation [21], (Table 2). In the study of Forestier et al., 1140 children aged 1-15 years were diagnosed with B-ALL from 1992 to 2004, from which 288 cases were positive for t(12;21) [8]. However, in the study of MA et al., who simultaneously investigated different ALL types like common, pre-B, early B- and T- for presence of t(12;21), only one case of T-ALL (7.5%) expressed this fusion [11].
In this study, the patient showed t(12;21) despite the presence of T-cell immunophenotype. The favorable prognosis of other ALL types harboring this translocation was also reported in this patient and we observed a good response to treatment in him until under follow up. As a result, the presence of t(12;21) may be observed in various ALL subgroups, which rules out its specificity for B-cells. Aberrant expression of B-cell and myeloid lineage markers in T-ALL complicates the diagnosis. Despite the fact that such cases rarely occur, they can significantly complicate leukemia diagnosis and survey of this group of patients requires an antibody panel for immunophenotypic markers.
Highlights
➢ CD19 and CD33can be expressed in T-ALL.
➢ Aberrant expression of t (12; 21), the specific B-ALL translocation, can occurinT-ALL.
➢ Investigation of aberrant gene rearrangement can be useful fordetermining the precise lineage of ambiguous ALL clones.
We wish to thank all our colleagues in Shafa Hospital and Allied Health Sciences School, Ahvaz Jundishapur University of Medical Sciences.
Ethical Approval
All procedures performed in this study were in accordance with the ethical standards of the local ethics committee of Ahvaz Jundishapur University of Medical Sciences and with the 1964 Helsinki declaration.
- Carroll WL, Bhojwani D, Min DJ, Raetz E, Relling M, et al. (2003) Pediatric acute lymphoblastic leukemia. ASH Education Program Book 2003: 102-131. Link: https://goo.gl/22BXq6
- Aguiar RC, Sohal J, Van Rhee F, Carapeti M, Franklin IM, et al. (1996) TEL‐AML1 fusion in acute lymphoblastic leukaemia of adults. Br J haematol 95: 673-677. Link: https://goo.gl/LmurGO
- Azad VF, Asl AAH, Tashvighi M, Mofrad NN, Haghighi M, et al. (2015) CD7 aberrant expression led to a lineage switch at relapsed childhood acute pre-B lymphoblastic leukemia. Med Mol Morphol 2015: 1-4. Link: https://goo.gl/GmxNf0
- Chessells JM, Harrison CJ, Kempski H, Webb DKH, Wheatley K, et al. (2002) Clinical features, cytogenetics and outcome in acute lymphoblastic and myeloid leukaemia of infancy: Report from the MRC childhood leukemia working party. Leukemia 16: 776-784. Link: https://goo.gl/N9PJoq
- Loh ML, McLean TW, Buckley JD, Howells W, Gilliland DG, et al. (1998) Lack of TEL/AML1 fusion in pediatric AML: further evidence for lineage specificity of TEL/AML1. Leuk Res 22: 461-464. Link: https://goo.gl/5ULguO
- Shurtleff SA, Buijs A, Behm FG, Rubnitz JE, Raimondi SC, et al. (1995) TEL/AML1 fusion resulting from a cryptic t(12;21) is the most common genetic lesion in pediatric ALL and defines a subgroup of patients with an excellent prognosis. Leukemia 9: 1985-1989. Link: https://goo.gl/o9J0nV
- Kempski H, Chalker J, Chessells J, Sturt N, Brickell P, et al. (1999) An investigation of the t(12;21) rearrangement in children with B- precursor acute lymphoblastic leukaemia using cytogenetic and molecular methods. Br J Haematol 105: 684-689. Link: https://goo.gl/uR5dBu
- Forestier E, Andersen MK, Autio K, Blennow E, Borgström G, et al. (2007) Cytogenetic patterns in ETV6/RUNX1‐positive pediatric B‐cell precursor acute lymphoblastic leukemia: A Nordic series of 245 cases and review of the literature. Genes, Chromosomes and Cancer 46: 440-450. Link: https://goo.gl/knuOA1
- Romana S, Poirel H, Leconiat M, Flexor M, Mauchauffe M, et al. (1995) High frequency of t (12; 21) in childhood B-lineage acute lymphoblastic leukemia. Blood 86: 4263-4269. Link: https://goo.gl/8tyyje
- Alvarez Y, Coll MD, Ortega JJ, Bastida P, Dastugue N, et al. (2005) Genetic abnormalities associated with the t(12;21) and their impact in the outcome of 56 patients with B-precursor acute lymphoblastic leukemia. Cancer Genetics and Cytogenetics 162: 21-29. Link: https://goo.gl/D2ZfLv
- Ma S, Wan T, Cheuk A, Fung L, Chan G, et al. (2001) Characterization of additional genetic events in childhood acute lymphoblastic leukemia with TEL/AML1 gene fusion: a molecular cytogenetics study. Leukemia 15: 1442-1447. Link: https://goo.gl/hXo0Ob
- Sawińska M, Ładoń D (2004) Mechanism, detection and clinical significance of the reciprocal translocation t(12;21)(p12;q22) in the children suffering from acute lymphoblastic leukaemia. Leukemia research 28: 35-42. Link: https://goo.gl/Y18ROX
- Harbott J, Viehmann S, Borkhardt A, Henze G, Lampert F (1997) Incidence of TEL/AML1 fusion gene analyzed consecutively in children with acute lymphoblastic leukemia in relapse. Blood 90: 4933-4937. Link: https://goo.gl/WZl8h1
- McLean TW, Ringold S, Neuberg D, Stegmaier K, Tantravahi R, et al. (1996) TEL/AML-1 dimerizes and is associated with a favorable outcome in childhood acute lymphoblastic leukemia. Blood 88: 4252-4258. Link: https://goo.gl/YdSFm4
- Seeger K, Adams H-P, Buchwald D, Beyermann B, Kremens B, et al. (1998) TEL-AML1 Fusion Transcript in Relapsed Childhood Acute Lymphoblastic Leukemia. Blood 91: 1716-1722. Link: https://goo.gl/4aAV1g
- Seeger K, Stackelberg AV, Taube T, Buchwald D, Körner G, et al. (2001) Relapse of TEL-AML1-positive acute lymphoblastic leukemia in childhood: A matched-pair analysis. J Clin Oncol 19: 3188-3193. Link: https://goo.gl/YtCJzN
- Liang DC, Chou TB, Chen JS, Shurtleff SA, Rubnitz JE, et al. (1996) High incidence of TEL/AML1 fusion resulting from a cryptic t(12;21) in childhood B-lineage acute lymphoblastic leukemia in Taiwan. Leukemia 10: 991-993. Link: https://goo.gl/wFCRR7
- Jahedi M, Shamsasenjan K, Sanaat Z, Aliparasti M, Almasi S, et al. (2014) Aberrant Phenotype in Iranian Patients with Acute Myeloid Leukemia. Adv Pharm Bull 4: 43-47. Link: https://goo.gl/zy9W97
- Jaffe ES (2001) Pathology and genetics of tumours of haematopoietic and lymphoid tissues: Iarc; 2001. Link: https://goo.gl/IDRnke
- Suggs JL, Cruse JM, Lewis RE (2007) Aberrant myeloid marker expression in precursor B-cell and T-cell leukemias. Exp Mol Pathol 83: 471-473. Link: https://goo.gl/IDHKlI
- Yao X, Teruya-Feldstein J, Raffeld M, Sorbara L, Jffe ES (2001) Peripheral T-cell lymphoma with aberrant expression of CD79a and CD20: a diagnostic pitfall. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc 14: 105-110. Link: https://goo.gl/Gd7lEK
- Rizzo K, Stetler‐Stevenson M, Wilson W, Yuan CM (2009) Novel CD19 expression in a peripheral T cell lymphoma: A flow cytometry case report with morphologic correlation. Cytometry B Clin Cytom 76: 142-149. Link: https://goo.gl/6ahzZp
- Matnani RG, Stewart RL, Pulliam J, Jennings CD, Kesler M (2013) Peripheral T-cell lymphoma with aberrant expression of CD19, CD20, and CD79a: case report and literature review. Case reports in hematology 2013. Link: https://goo.gl/iCd68a
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Help ?
Help ?

If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."

 Save to Mendeley
Save to Mendeley