International Journal of Immunotherapy and Cancer Research
Immunogenicity in CAR T cell immunotherapy
Student at Westview High School, USA
Author and article information
Cite this as
Yu E (2023) Immunogenicity in CAR T cell immunotherapy. Int J Immunother Cancer Res. 2023; 9(1): 013-016. Available from: 10.17352/2455-8591.000038
Copyright License
© 2023 Yu E. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Currently, the most accessible forms of cancer treatment include surgery, chemotherapy, and radiation. However, these forms of treatment may damage or destroy healthy tissue as well as cancerous cells, resulting in side effects such as fatigue, hair loss, diarrhea, etc. Immunotherapy, an alternative form of cancer treatment, is a growing treatment method of interest that uses bodily substances made by the body or in a laboratory to boost the immune system’s activity against tumor cells. One type of immunotherapy is CAR T cell therapy, in which a patient’s T cells are genetically modified in a lab to express Chimeric Antigen Receptors (CARs) that help T cells identify and destroy their target. However, because CARs are constructed in the lab and currently consist of non-self components, genetically engineered CAR T cells have the potential to induce anti-CAR immune responses. The following paper will explore the causes of anti-CAR immunity, its possible solutions, and the potential implications of these discoveries.
CAR: Chimeric Antigen Receptor; scFv: single chain variable Fragment; VH: Variable Heavy; VL: Variable Light; MHC: Major Histocompatibility Complex; APC: Antigen-Presenting Cell; TCR: T Cell Receptor; HLA: Human Leukocyte Antigen; NK cells: Natural Killer cells; CDR: Complementarity-Determining Region; FR: Framework Region
Introduction
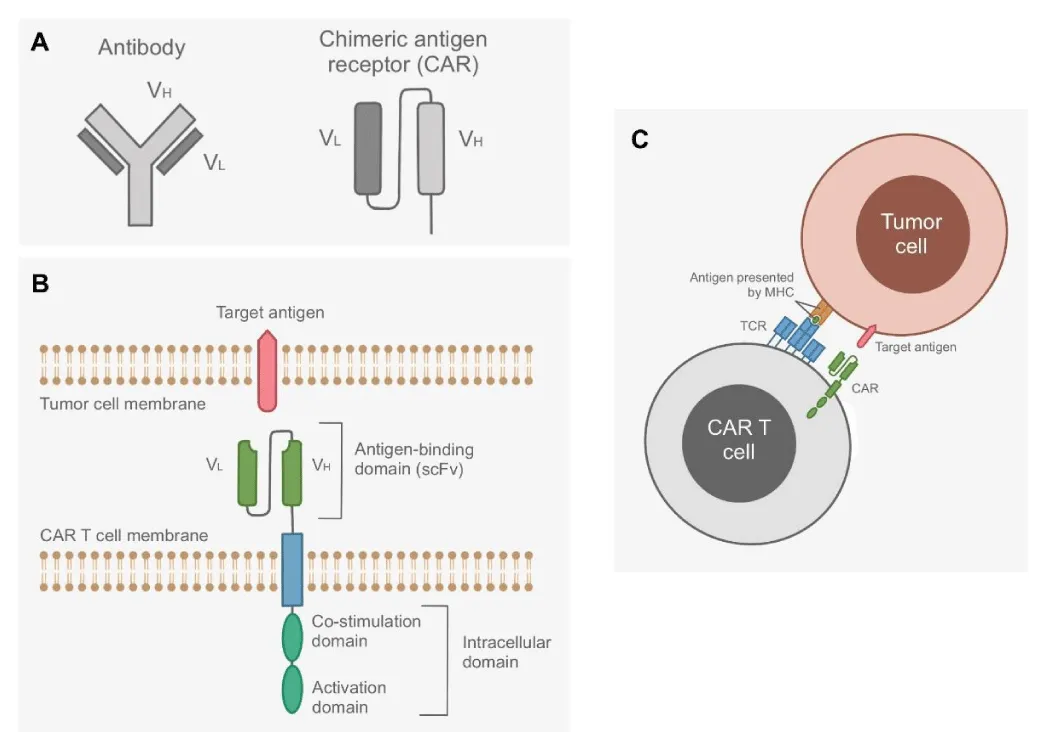
Chimeric Antigen Receptors (CARs) are recombinant receptors that are expressed on genetically modified CAR T cells to promote T cell recognition and destruction of tumorous cells. CARs are made of two parts: an antigen-binding single chain Variable Fragment (scFv) and an intracellular signaling molecule. The scFv is typically made of the Variable Heavy (VH) and Variable Light (VL) chains of an antibody. The intracellular portion consists of a signaling chain that couples antigen recognition to intracellular signal-transduction pathways and optional co-stimulation domains [1]. Different CARs are recombined to recognize specific antigens presented on tumorous cells that the body’s normal T cells may not identify as foreign [2].
CAR T cell immunotherapy results in immunogenicity
Because current methods of artificially constructing CARs use non-self fragments, immunogenicity is a critical contributor to the lower efficacy and success rates of administered CAR T cells. The body may induce anti-CAR immune responses to non-self components of the CAR constructs or to residual proteins from the inherently immunogenic gene-transfer vectors that are used in the process of genetically engineering CAR T cells [3,4].
The body’s natural immune response may reject administered CAR T cells through both cellular and humoral anti-CAR responses. Cellular immunity likely arises from the cross-presentation of foreign sequences in the CAR molecule by a Major Histocompatibility Complex (MHC). When CAR T cells naturally die through apoptosis, foreign mice-derived scFv fragments may be displayed through MHC molecules by Antigen-Presenting Cells (APCs) and used to prime T cell responses, thereby turning the body’s T cells against the CAR molecule. Humoral immunity is also primed through CAR proteins, but the foreign CAR construct fragments are instead presented by follicular dendritic cells to B cells. CAR-specific B cells can then undergo plasma cell differentiation and class switching, in which B cells change the type of antibodies they produce, thereby enabling the immune system to manufacture anti-CAR antibodies that induce the death of CAR T cells [5].
Although initial clinical administrations of CAR T cells have high response rates, studies have noted an increase in the amount of detected anti-CAR antibodies following infusion [6] and disease relapse after the first round of CAR T cell treatment [7]. These results suggest that anti-CAR immunity is a prevalent issue that significantly impacts the efficacy of CAR T cell immunotherapy. Fortunately, there are a few, promising methods that have the potential to combat anti-CAR immune responses (Figure 1).
Lymphodepletion
One method to minimize anti-CAR immune responses is lymphodepletion, in which the number of immune cells that have the potential to attack CAR T cells is decreased. The primary method of lymphodepletion is through chemotherapy; fludarabine and cyclophosphamide are recognized as the most promising chemotherapy medications so far [9]. Fludarabine interferes with ribonucleotide reductase and DNA polymerase to inhibit DNA synthesis [10] and cyclophosphamide forms cross-linkages within and between DNA strands at the guanine N-7 position, resulting in permanent modifications that lead to programmed cell death [11]. The combined use of fludarabine and cyclophosphamide is currently the most commonly used combination for inducing immune cell death before CAR T cell treatment therapy. Some patients, though, fail to develop a favorable immune environment that is inhibited by lymphodepletion even with the best lymphodepletion regime, suggesting that the effectiveness of lymphodepletion chemotherapy also depends on the host biological response [9].
Current methods of lymphodepletion chemotherapy come with harmful side effects, however, including pancytopenia and prolonged immune suppression. Pancytopenia is a condition characterized by low levels of red blood cells, white blood cells, and platelets. This consequently leads to greater chances of anemia, infection, and excessive bruising or bleeding [12]. Prolonged immune suppression may be caused by the lingering effects of fludarabine and cyclophosphamide after lymphodepletion is no longer necessary, thereby increasing the risk of infections. Additionally, fludarabine can induce neurotoxicity and fever, while cyclophosphamide can induce hemorrhagic cystitis and pericarditis, and both may increase the risk of secondary malignancies [9]. Although lymphodepletion has the potential to increase the efficiency of CAR T cell therapy, the side effects of current treatment methods warrant further research into the mechanism of immune suppression by chemotherapy medication.
Elimination of MHC molecules on CAR T cells
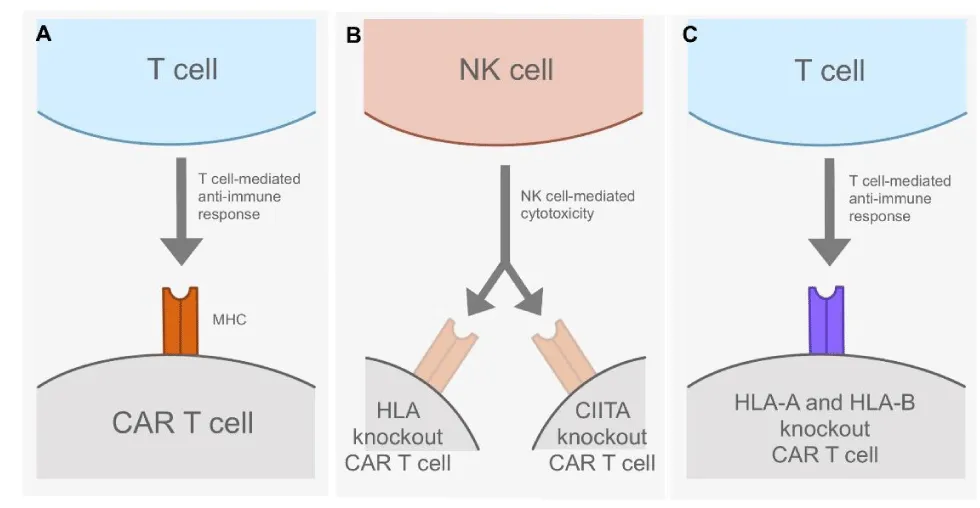
Another proposed method to combat anti-CAR T cell immune responses takes advantage of programmable nucleases that use the CRISPR-Cas9 genome editing system. Researchers can use gene editing to eliminate cell-surface MHC expression on CAR T cells and thereby prevent the detection and elimination of CAR T cells by T cell-mediated anti-CAR immunity. Researchers first tried to remove MHC I from the surface of resting CAR T cells, as MHC I molecules are expressed on all nucleated cells and play a crucial role in alerting the immune system to infected cells [13]. Eliminating surface MHC I expression indeed prevents alloimmune reactions, but it requires the genetic editing of the human leukocyte antigen (HLA) locus, which are genes in MHCs that help code for proteins that differentiate between self and non-self [14]. The eradication of HLA expression would consequently increase natural killer (NK) cell-mediated cytotoxicity. A potential way to combat this issue is to prevent the expression of HLA-A and HLA-B while allowing the expression of HLA-C, an MHC component that is assumed to act as a ligand for killer immunoglobulin receptors expressed on NK cells and may minimize NK cell-mediated cytotoxicity [15].
MHCs may also be eliminated from the cell surface by disrupting the functional expression of CIITA, which encodes the master transcriptional regulator of MHC II [16]. The inhibition of CIITA expression would remove MHC II from the cell surface without provoking alloimmune responses to activated CAR T cells. However, this method again introduces the issue of NK cell-mediated cytotoxicity [5]. No matter what approach is used to eliminate MHC surface-expression levels, it is evident that the identification of foreign cells by the immune system via MHCs is still an ambiguous area of study that, if clarified, could increase the efficacy of CAR T cell therapy (Figure 2).
Development of CAR constructs with humanized scFv
The most critical issue facing current CAR T cell therapy methods, however, is the development of CARs from scFv fragments that are derived from mice. When CAR T cells die through apoptosis, the foreign fragments in CAR constructs are displayed by MHC molecules expressed on APCs, leading to an anti-CAR T cell immune response.
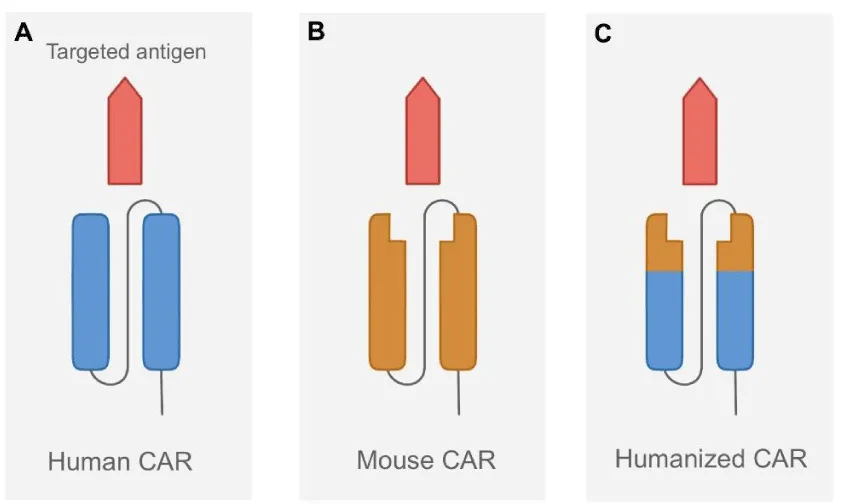
To combat this problem, researchers are looking at the possibility of using humanized CAR constructs, which would be less immunogenic than CARs constructed from mouse-derived scFvs. The humanization of scFvs requires the execution of Complementarity-Determining Region (CDR) grafting with the retention of mouse Framework Region (FR) residues to ensure that the novel humanized constructs maintain the same function and efficacy as the ones previously constructed with mouse scFv fragments. To generate the humanized scFv gene, CDRs of the mouse VH and VL regions are grafted onto selected human FRs that show the highest similarity to the amino acid sequence identity of the FRs of mouse VH and VL [17].
MHC molecules may be reconstructed using entirely human-derived CAR constructs, or they may have traditional scFvs substituted with immunoglobulin heavy-chain-only recognition domains that lack light chains and potential immunogenic linker sequences. Because linkers are another possible source of immunogenicity, the elimination of linker sequences from CARs would be beneficial. These heavy-chain CAR constructs have shown significant target affinity and efficacy in preclinical models, but constructs that are derived solely from self-human components could still theoretically initiate immune responses in patients [18]. Further head-to-head clinical trials directly comparing the immunogenicity and efficacy of mouse-derived versus humanized CAR constructs are necessary, and if humanized CARs indeed have higher efficacy and lower immunogenicity, they would lead a promising direction for the creation of commercial CAR T cell products. This would make CAR T cell therapy both more convenient and less expensive [19] (Figure 3).
Conclusion
Although immunogenicity in CAR T cell immunotherapy is a pressing issue, there are developing methods that have the potential to combat it. Studying patients’ varied responses to fludarabine and cyclophosphamide would allow chemotherapy treatment to eliminate the immune cells that attack administered CAR T cells without downregulating the immune system for an extended period of time. Researching the many caveats of MHC-facilitated immune cell recognition may prevent CAR T cells from being recognized and therefore targeted by the body’s immune system. The replacement of mouse-derived scFvs with humanized ones can create CAR constructs that are less likely to stimulate anti-CAR immune responses, but further clinical trials are required to test the constructs’ immunogenicity. Finding the solution to immunogenicity in CAR T cell therapy is undoubtedly a difficult process, but it would significantly improve the efficacy of CAR T cell therapy and open the doors of immunotherapy treatment to a plethora of new possibilities.
- Feins S, Kong W, Williams EF, Milone MC, Fraietta JA. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am J Hematol. 2019 May;94(S1):S3-S9. doi: 10.1002/ajh.25418. Epub 2019 Feb 18. PMID: 30680780.
- CAR T-cell Therapy and Its Side Effects. (2022. https://www.cancer.org/cancer/managing-cancer/treatment-types/immunotherapy/car-t-cell1.html
- Riddell SR, Elliott M, Lewinsohn DA, Gilbert MJ, Wilson L, Manley SA, Lupton SD, Overell RW, Reynolds TC, Corey L, Greenberg PD. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat Med. 1996 Feb;2(2):216-23. doi: 10.1038/nm0296-216. PMID: 8574968.
- Lamers CH, Willemsen R, van Elzakker P, van Steenbergen-Langeveld S, Broertjes M, Oosterwijk-Wakka J, Oosterwijk E, Sleijfer S, Debets R, Gratama JW. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011 Jan 6;117(1):72-82. doi: 10.1182/blood-2010-07-294520. Epub 2010 Oct 1. PMID: 20889925.
- Wagner DL, Fritsche E, Pulsipher MA, Ahmed N, Hamieh M, Hegde M, Ruella M, Savoldo B, Shah NN, Turtle CJ, Wayne AS, Abou-El-Enein M. Immunogenicity of CAR T cells in cancer therapy. Nat Rev Clin Oncol. 2021 Jun;18(6):379-393. doi: 10.1038/s41571-021-00476-2. Epub 2021 Feb 25. PMID: 33633361; PMCID: PMC8923136.
- Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, Robinson E, Steevens NN, Chaney C, Soma L, Chen X, Yeung C, Wood B, Li D, Cao J, Heimfeld S, Jensen MC, Riddell SR, Maloney DG. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016 Jun 1;126(6):2123-38. doi: 10.1172/JCI85309. Epub 2016 Apr 25. PMID: 27111235; PMCID: PMC4887159.
- Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019 Jun;16(6):372-385. doi: 10.1038/s41571-019-0184-6. PMID: 30837712; PMCID: PMC8214555.
- Maakaron JE, Hu M, El Jurdi N. Chimeric antigen receptor T cell therapy for cancer: Clinical applications and practical considerations. BMJ. 2022: e068956. https://doi.org/10.1136/bmj-2021-068956
- Kro¨ger N, Gribben J, Chabannon C, Yakoub-Agha I, Einsele H. The EBMT/EHA CAR-T cell handbook. Springer. 2022.
- Ricci F, Tedeschi A, Morra E, Montillo M. Fludarabine in the treatment of chronic lymphocytic leukemia: a review. Ther Clin Risk Manag. 2009 Feb;5(1):187-207. doi: 10.2147/tcrm.s3688. Epub 2009 Mar 26. PMID: 19436622; PMCID: PMC2697528.
- Ogino MH, Tadi P. (n.d.). Cyclophosphamide. StatPearls.
- Pancytopenia. (2023). https://my.clevelandclinic.org/health/diseases/25105-pancytopenia
- Hewitt EW. The MHC class I antigen presentation pathway: strategies for viral immune evasion. Immunology. 2003 Oct;110(2):163-9. doi: 10.1046/j.1365-2567.2003.01738.x. PMID: 14511229; PMCID: PMC1783040.
- Choo SY. The HLA system: genetics, immunology, clinical testing, and clinical implications. Yonsei Med J. 2007 Feb 28;48(1):11-23. doi: 10.3349/ymj.2007.48.1.11. PMID: 17326240; PMCID: PMC2628004.
- Blais ME, Dong T, Rowland-Jones S. HLA-C as a mediator of natural killer and T-cell activation: spectator or key player? Immunology. 2011 May;133(1):1-7. doi: 10.1111/j.1365-2567.2011.03422.x. Epub 2011 Mar 1. PMID: 21355865; PMCID: PMC3088962.
- Devaiah BN, Singer DS. CIITA and Its Dual Roles in MHC Gene Transcription. Front Immunol. 2013 Dec 20;4:476. doi: 10.3389/fimmu.2013.00476. PMID: 24391648; PMCID: PMC3868913.
- Khantasup K, Chantima W, Sangma C, Poomputsa K, Dharakul T. Design and Generation of Humanized Single-chain Fv Derived from Mouse Hybridoma for Potential Targeting Application. Monoclon Antib Immunodiagn Immunother. 2015 Dec;34(6):404-17. doi: 10.1089/mab.2015.0036. PMID: 26683180; PMCID: PMC4685505.
- Cao J, Wang G, Cheng H, Wei C, Qi K, Sang W, Zhenyu L, Shi M, Li H, Qiao J, Pan B, Zhao J, Wu Q, Zeng L, Niu M, Jing G, Zheng J, Xu K. Potent anti-leukemia activities of humanized CD19-targeted Chimeric antigen receptor T (CAR-T) cells in patients with relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. 2018 Jul;93(7):851-858. doi: 10.1002/ajh.25108. Epub 2018 Apr 28. PMID: 29633386.
- Levine BL, Miskin J, Wonnacott K, Keir C. Global Manufacturing of CAR T Cell Therapy. Mol Ther Methods Clin Dev. 2016 Dec 31;4:92-101. doi: 10.1016/j.omtm.2016.12.006. PMID: 28344995; PMCID: PMC5363291.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
