International Journal of Immunotherapy and Cancer Research
Pro-inflammatory cytokine and vascular adhesion molecule levels in manganese and lead-exposed workers
Ozgur Oztan1, Vugar Ali Turksoy2*, Iskender Samet Daltaban3, Meside Gunduzoz4, Lutfiye Tutkun5, Servet Birgin Iritas6 and Hakan AK3
2Department of Public Health, Yozgat Bozok University Faculty of Medicine, Yozgat, Turkey
3Department of Brain and Nerve Surgery, Yozgat Bozok University Faculty of Medicine, Yozgat, Turkey
4Department of Family Medicine, Ankara Occupational Diseases Hospital Ankara, Turkey
5Department of Nutrition and Dietetics, Gazi University Health Science Faculty, Ankara, Turkey
6The Council of Forensic Medicine, Ministry of Justice, Ankara, Turkey
Cite this as
Oztan O, Turksoy VA, Daltaban IS, Gunduzoz M, Tutkun L, et al. (2019) Pro-inflammatory cytokine and vascular adhesion molecule levels in manganese and lead-exposed workers. Int J Immunother Cancer Res 5(1): 001-007. DOI: 10.17352/2455-8591.000020Objectives: This study aimed to develop a model of the relationship between inflammatory cytokine and/or vascular adhesion molecule levels and clinical symptoms in individuals exposed to lead (Pb), manganese (Mn) or both (Pb+Mn).
Methods: The study included 104 male workers exposed to metals for different durations in a factory setting (Mn-, Pb- and Pb+Mn-exposed groups) and 76 non-exposed male workers (control group). Interleukin (IL)-6, IL-10, Tumor Necrosis Factor (TNF)-α, soluble (s)E-selectin, and Vascular Cell Adhesion protein (VCAM)-1 levels were analyzed using enzyme-linked immunosorbent assays. Mn and Pb levels were determined using inductively coupled plasma mass spectrometry.
Results: Significant intergroup differences were observed in the levels of IL-6, IL-10, TNF-α, and sE-selectin (p<0.01 for all), but not VCAM-1 (p=0.298). Fatigue was the most frequent symptom in all groups (25.7%, 37.1%, and 44.1%, respectively). Other symptoms included tremor and anxiety in the Mn-exposed group (5.7%), and tremor and paresthesia in the Pb-exposed (14.7%) and Pb+Mn-exposed groups (20.6%).
Conclusions: The correlation between increased biomarker levels and clinical symptoms suggests a close relationship between inflammation and neurotoxicity. This relationship not only explains the effect of the former on the latter but also provides a clinical model for the early diagnosis of neuroinflammation.
Introduction
Constitutive neurogenesis affects both children and adults and is known to correlate closely with neurocognitive functions such as learning and memory [1]. Inflammation is a systemic reaction. However, the brain reacts to inflammation differently than peripheral tissues. Under normal conditions, the Blood-Brain Barrier (BBB) is selectively permeable and allows only the passage of T-cells, macrophages, and dendritic cells [2]. However, inflammation of the brain initiates the secretion of anti- and pro-inflammatory cytokines by resident microglia, astrocytes, infiltrating peripheral macrophages, and lymphocytes. This activity both stimulates the migration of leukocytes to the region and promotes astrogliosis [3].
Pathologically, Parkinson’s Disease (PD) is characterized by the massive degeneration and loss of nigrostriatal dopaminergic neurons. Many studies of PD have also emphasized the concomitant neuroinflammation and increased oxidative stress resulting from the presence of inflammatory markers [4,5]. Pathologically, the activation of microglial cells is considered the initial and significant inflammatory response and is associated with increased levels of cytokines, especially Tumor Necrosis Factor (TNF)-α, interleukin (IL)-6, and IL-10 [6,7]. Among the approximately 200 cytokines that have been recognized to date, IL-6, IL-10, and TNF-α are well known to play pivotal roles in inflammation. IL-10 appears to moderate the inflammatory response by inhibiting the expression of many inflammatory cytokines, chemokines, and chemokine receptors [8] and also suppresses the pro-inflammatory functions of cytokines such as TNF-α, IL-6, IL-1, and IL-8 [9]. IL-6 regulates immune responses, including inflammation, by promoting T-cell proliferation, B-cell differentiation, and IgG, IgA, and IgM production by plasma cells [10]. The pro-inflammatory cytokine TNF-α contributes directly or indirectly to the degeneration of dopaminergic neurons in the substantia nigra [11,12].
Meanwhile, Vascular Cell Adhesion Molecule (VCAM)-1 and E-selectin are known to accelerate the adhesion and transmigration of mononuclear cells through the vascular endothelium, including the BBB [13,14]. These adhesion molecules have significant predictive value for clinical monitoring. Accordingly, many studies of cerebrovascular diseases, including stroke and cerebral infarction, identified VCAM-1 and E-selectin as potential biomarkers for various stages of these diseases [15,16].
Manganese (Mn) is a cofactor for many metabolic enzymes and is thus essential for life. In the brain, Mn plays vital roles in the synthesis of manganese superoxide dismutase and glutamine synthetase [17,18]. Despite these essential roles, Mn overexposure is common, especially in occupational and environmental settings. Occupational exposure occurs most frequently among welders, miners, and metal recyclers [19]. According to animal studies, Mn was shown to accumulate in the brains of rats during the first 4 days of administration, and its primary targets were the globus pallidus and striatum of the basal ganglia. Furthermore, from the brain at a much slower rate than from other tissues, such as the liver and kidney [20,21].
Mn overexposure and related neurotoxicity may cause the development of manganism, a parkinsonian syndrome. Despite significant clinical similarities between manganism and Idiopathic Parkinson’s Disease (IPD), cumulative evidence shows that manganism primarily affects the globus pallidus and striatum of basal ganglia, whereas IPD mainly targets the substantia nigra [22]. Additionally, some studies have reported a link between occupational exposure to Mn and disorders of seminal functioning and hormone secretion [23,24].
Lead (Pb), a toxic metal with no identified biological function, has been the cause of widespread occupational and environmental contamination and therefore, lead exposure has become a major public health issue. Several mechanisms have been proposed to describe the role of Pb in the development of neurotoxicity, and acute or chronic Pb exposure has been shown to cause several neurological problems, including neurobehavioral functional impairment, peripheral neuropathies, and chronic encephalopathy [25-27]. Similar to Mn exposure, several studies have linked an increase in inflammatory biomarkers to Pb neurotoxicity [28,29]. Furthermore, many disorders, such as hypertension, atherosclerosis, anemia, renal impairment, and hearing loss, were found to be associated with chronic Pb exposure in an occupational setting [30,31].
Therefore, in this study, we aimed to develop a model that would elucidate the relationship of pro-inflammatory cytokine and/or adhesion molecule levels with the severity of neurological conditions in individuals exposed to Pb, Mn, or both metals (Pb+Mn).
Materials and methods
Study population
This study included 104 male workers who were exposed to Mn, Pb, or Pb+Mn in the workplace and 76 male workers with no history of occupational exposure to these or other metals (control group). Both control and exposed subjects were examined at the Ankara Occupational and Environmental Diseases Hospital in 2017. For all subjects, the age and body mass index were determined. Information about cigarette smoking, alcohol consumption, and chronic diseases was obtained through self-reported questionnaires.
The exposed group comprised welders and metal recyclers who were identified by their work histories and the results of toxicological analyses. The inclusion criteria for the exposed groups were a high serum level of Mn (Group 2, n=35), Pb (Group 3, n=35), or Pb+Mn (Group 4, n=34). Overexposure was defined as a blood or Mn level exceeding 5 or 10μg/dL, respectively [32,33]. Subjects with conditions that might affect biomarker levels, including coronary vascular disease, hypertension, rheumatic diseases, diabetes mellitus, acute infections, chronic lung disease, cancer, and alcohol addiction, were excluded from the study. All subjects in the control group were healthy and asymptomatic and were selected from among nonsmoking and non-alcohol-consuming workers to eliminate the effects of smoking and alcohol consumption on symptoms attributable to neurotoxicity.
Collection and laboratory analysis of biological samples
During annual health examinations, blood samples were collected from each subject for immunological, toxicological, and routine analyses. Serum was separated from each blood sample via centrifugation at 1500 rpm for 10 minutes and transferred to 2mL centrifuge tubes. Subsequently, the serum samples were transported to the Occupational and Environmental Toxicology Laboratory of the Yozgat Bozok University Science and Technology Application and Research Center (BILTEM) in a cold chain environment. All samples were stored at -20°C until analysis.
The serum levels of IL-6, IL-10, TNF-α, sE-selectin, VCAM-1, Mn, and Pb levels were assessed in diluted samples. The levels of IL-6, IL-10, TNF-α, sE-selectin, and VCAM-1 were determined using Enzyme-Linked Immunosorbent Assay (ELISA) kits (KAP1261, KAP1321, and KAP1751 from DIAsource, Belgium; DZE201120263 and DZE201120204 from SunRed-bio, Shanghai, respectively). The samples were placed on ELISA microplates pre-treated according to the kit instructions. Once the assay reactions were terminated, the results were determined using a plate reader (CLARIOstar, BMG LABTECH). For all cytokine measurements, a 450nm wavelength and 5-point calibration curves were used (respective r2 values: 0.9995, 0.9993, 0.9994, 0.9997, and 0.9992). Quality control samples were used for verification. A linear regression was used to evaluate all cytokine data.
To analyze Mn and Pb, 1-mL aliquots of blood were placed in Teflon tubes in a microwave oven (Start D Microwave Digestion System, Milestone). Thereafter, 5mL of nitric acid (HN03 65%, Suprapur) and 5mL of ultrapure water were added to the blood samples, which were digested in the microwave. The samples were then transferred to 50mL polypropylene tubes, and deionized water was added to a total volume of 20mL. The digested samples were stored in these tubes at 4°C prior to analysis [34]. Subsequently, the samples were subjected to inductively coupled plasma mass spectrometry (iCAP Qc, Thermo Scientific, USA). The method was validated using Certified Reference Materials (Seronorm™ Trace Elements Whole Blood L-2). The r2 values of the calibration curves were found 0.9999 (f(x)=106685.2562*×+115396.5787; BEC=1.082ppb; LoD=0.0860ppb) for Mn and 0.9999 (f(x)=344403.6097*×+45469.3049; BEC=0.132ppb; LoD=0.0084ppb) for Pb.
Statistical analysis
SPSS Statistics version 24.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. The relationships between the main parameters and symptoms in the control and exposed groups were evaluated using the t-test (Tables 1,2). Associations between categorical variables were evaluated using the chi-square test (Table 2). The Pearson test was performed to evaluate the correlations between cytokine levels and toxicological/biochemical variables in all groups (Table 3). A regression analysis was used to identify the independent variables (Mn and Pb) that correlated with the dependent variable (IL-6, TNF-a, sE-selectin) (Table 4). For all analyses, a p-value <0.05 was considered statistically significant.
Results
This study included 180 male subjects, who were stratified into a control group (Group 1) of 76 subjects, Mn-exposed group (Group 2) of 35 subjects, Pb-exposed group (Group 3) of 35 subjects, and Pb+Mn-exposed group (Group 4) of 34 subjects. The relationships between the main parameters in all groups are presented in Table 1. Participants in Groups 1, 2, 3, and 4 reported work durations of mean±standart deviation as 15.49±6.46, 15.80±5.71, 15.17±3.30, and 14.53±4.67 years, respectively. The subjects were young and middle-aged, with no significant differences in age between the groups (42.45±7.27, 42.74±7.20, 42.14±5.88, and 41.74±6.97 years, for Groups 1, 2, 3, and 4; p<0.936) (Table 1). However, significant differences in Mn and Pb levels (p<0.001 for all) were observed between the groups (Table 1).
In our study, the serum levels of IL-6, IL-10, TNF-α, and sE-selectin were significantly higher in Mn-exposed subjects than in control subjects (for IL-6: 38.33±11.93 vs. 30.02±16.70pg/mL; for IL-10: 44.46±15.94 vs. 34.30±18.54pg/mL; for TNF-α: 4.40±1.45 vs. 3.58±1.35pg/mL; for sE-selectin: 58.25±20.23 vs. 41.78±19.55pg/mL; p<0.01 for all). Similarly, the levels of these biomarkers were also significantly higher in Pb-exposed and Pb+Mn-exposed subjects than in control subjects (for IL-6: 35.41±8.73, 42.57±21.12, and 30.02±16.70pg/mL; for IL-10: 40.21±3.73, 50.56±20.90, and 34.30±18.54pg/mL; for TNF-α: 4.66±0.34, 5.27±1.52, and 3.58±1.35pg/mL; for sE-selectin: 47.47±9.71, 54.63±13.52, and 41.78±19.55ng/mL, respectively; p<0.01 for all). On the other hand, there was no significant difference in VCAM-1 levels for all groups (7.82±1.55ng/mL in the Pb+Mn-exposed group, 6.99±1.52ng/mL in the Pb-exposed group, 7.08±2.39ng/mL in the Mn-exposed group, and 8.04±4.41ng/mL in the control group; p>0.05 for all) (Table 1). Moreover, a comparison among the symptomatic groups (excluding the control group) revealed significant differences in IL-6 and IL-10 levels (p<0.01), but not in TNF-α and sE-selectin levels (p>0.05) (Table 2).
The Pearson correlation coefficients of all parameters are presented in Table 3. Significant highly positive correlations were observed between the level of Mn and the levels of IL-6, TNF-α, and sE-selectin (r=0.229, r=0.206, and r=0.297, respectively; p<0.01). However, a negative correlation was found between the Mn and hemoglobin levels (r=-0.155, p<0.01). Furthermore, a significant highly positive correlation was observed between the Pb and TNF-α levels (r=0.200, p<0.01). The TNF-α level also correlated positively with the white blood cell count (r=0.184, p<0.05), while the VCAM-1 level correlated positively with the hematocrit level (r=0.148, p<0.05) (Table 3).
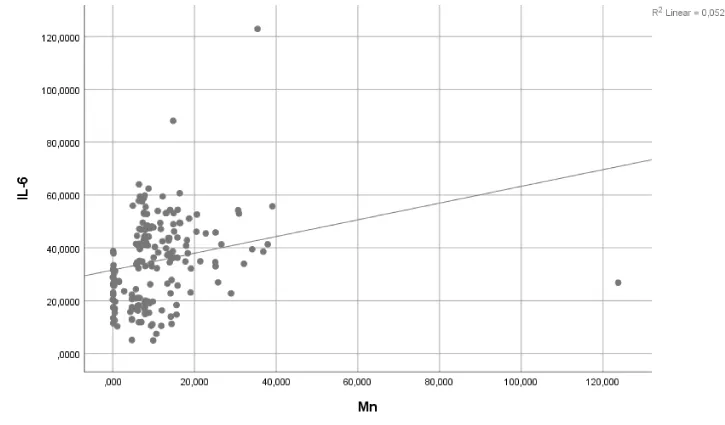
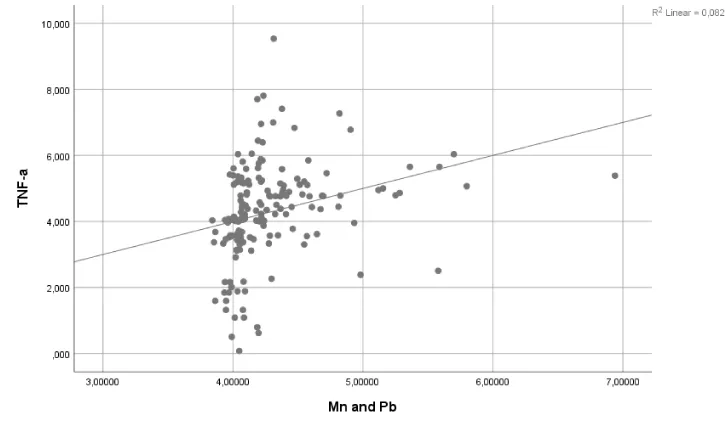
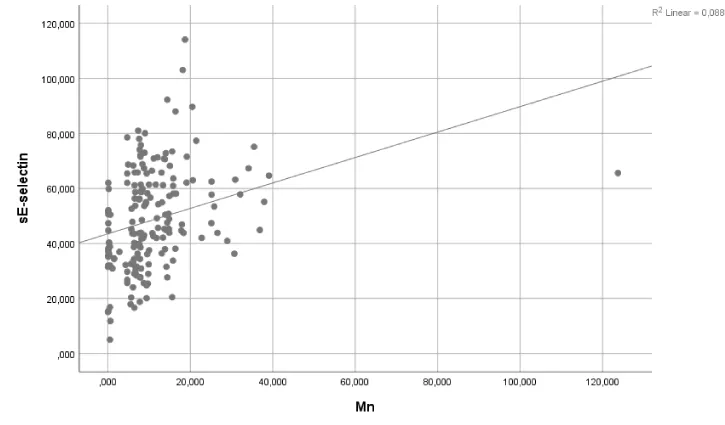
A regression analysis revealed no significant effects of Pb exposure on IL-6 and sE-selectin levels (p>0.05) (Table 4). Similarly, Pb+Mn exposure had no effect on IL-10 levels (p>0.05). However, Mn exposure accounted for 5.2% and 8.8% of the observed changes in IL-6 and sE-selectin levels, respectively (p<0.05 and p<0.01, respectively). Pb+Mn exposure accounted for 8.2% of the observed changes in TNF-a (p<0.05). The results of the regression analysis are shown in Table 4 and the corresponding graphs are presented in Figures 1-3.
Discussion
Central nervous system inflammation can cause significant brain damage [35]. The pathogenesis of various neurological diseases, including PD, Alzheimer’s disease, multiple sclerosis, and dementia, is closely related to localized inflammatory responses in the brain. The activation of nigral microglia and the release of pro-inflammatory neurotoxic factors may be an essential component of the process of dopaminergic neuron degeneration in PD [36-39]. Although chronic low-to-high-dose Mn exposure may cause a neurotoxic syndrome that is symptomatically similar to PD, the former syndrome lacks nigrostriatal dopaminergic neuron damage and thus differs from clinical PD. Additionally, this neurotoxic syndrome is resistant to levodopa treatment [40]. Therefore, an incorrect assessment of the patient’s Mn exposure history may lead to difficulties in diagnosis and treatment.
Pb is a neurotoxic substance with well-known deleterious neurodevelopmental effects in children. Pb also plays an important role in the etiology and pathogenesis of many diseases ranging from peripheral neuropathy to acute encephalopathy. Pb exposure also presents a diagnostic challenge because it tends to be associated with slight neurological symptoms. For this reason, we aimed to develop a model demonstrating the relationship between the levels of inflammatory cytokines and/or vascular adhesion molecules and the presence or absence of clinical symptoms in individuals exposed to Pb, Mn, or both. Although numerous studies have evaluated the link between toxic metal exposure and neurotoxicity, few have investigated the association between pro-inflammatory cytokine levels and neurotoxicity in such cases.
In this study, exposure symptoms were observed in 11, 18, and 19 subjects in Groups 2, 3, and 4, respectively. In all exposed groups, the most common symptom was fatigue (25.7%, 37.1%, and 44.1% of 104 subjects, respectively), while the least frequent symptoms were tremor and anxiety in Group 2(5.7%) and tremor and paresthesia in Groups 3(14.3%) and 4(20.6%). Bowler et al. observed that tremors (91.5%) was the most frequently reported symptom in a cohort of welders, followed by mood changes (72.3%), neurological problems (61.7%), sleep disturbances (55.3%), headaches (44.7%), and sexual dysfunction (28%) [41]. By contrast, our study observed considerably lower frequencies of these symptoms, suggesting that the Mn-exposed subjects might have been in the initial phases of neurotoxicity. Fatigue and depression were the most frequent symptoms among Mn-exposed subjects (25.7% and 20.0%, respectively), while fatigue and headache were the most frequent symptoms in the Pb+Mn-exposed group (44.1% and 32.4%, respectively).
Manganism is a neurodegenerative disease of the cerebral cortex and basal ganglia caused by excessive exposure to Mn [42,43]. Neuroinflammatory activation plays roles in both the progression of manganism and the promotion of neurological damage from both acute and chronic exposure. Although TNF-α is the main regulator of multiple inflammatory genes, IL-6 and CCL5 were shown to regulate the signals and production of these genes in two types of glial cells during Mn exposure [44]. Cytokines have been increasingly implicated in acute and chronic neuronal degeneration [45]. Recent clinical studies have reported increased levels of TNF-α, IL-6, IL-1b, and interferon-γ in post-mortem brain tissues [46,47]. In our study, we observed strong relationships between the levels of Mn and of the pro-inflammatory cytokines IL-6 and TNF-α, as well as the adhesion marker sE-selectin. We also identified associations between the levels of IL-6 and IL-10 levels and clinical symptoms. By contrast, Scharrer et al. reported no changes in the blood levels of fibrinogen, C-reactive protein, antithrombin III, factor VIII, von Willebrand factor, soluble intercellular adhesion molecule-1, TNF-α, IL-6, and IL-8 in subjects exposed to welding fumes [48]. Previous studies have reported significant increases in the levels of major neuroinflammatory cytokines, such as TNF-α, after Mn exposure in a dose-dependent manner. Additionally, most previous studies reported strong correlations of the blood levels of cytokines and vascular adhesion molecules with the intensity of exposure [46]. In our study, the serum levels of IL-6, IL-10, TNF-α, and sE-selectin were significantly higher in Mn-, Pb-, and Pb+Mn-exposed subjects, compared to control subjects. However, we observed no significant differences in VCAM-1 levels between the groups.
Importantly, our study findings describe the characteristics of the exposed groups. All subjects were young and middle-aged male workers. The exposed subjects exhibited neurological symptoms that correlated strongly with the neurotoxic effects of Mn and Pb exposure. We note that continued exposure in the absence of proper protection could enhance the severity and frequency of clinical symptoms as the affected individual ages. Therefore, our findings indicate the strong relationship between healthy working conditions and healthy aging.
Conclusions
In conclusion, our study revealed an association of symptoms attributable to Pb, Mn and Pb+Mn exposure with inflammation. Exposure to these metals was responsible for activating the inflammatory response, which in turn was associated with increases in the levels of cytokines (IL-6 and TNF-α) and sE-selectin. The subjects’ conditions also correlated significantly with symptoms associated with increased levels of pro-inflammatory cytokines, especially IL-6 and IL-10. The identified correlations between increased biomarker levels and clinical symptoms suggest a close relationship between inflammation and neurotoxicity. Our findings not only support the concept of inflammation as an etiological mechanism underlying neurotoxicity but also provide clinicians with a model for the early diagnosis of neuroinflammation.
To our knowledge, this is the first published report of an evaluation of these biomarkers in the context of combined Pb+Mn exposure. Although several reports have focused separately on the etiological relationships of Pb and Mn with neurotoxicity, combined exposure is very common and thus comprises a major health problem, especially in occupational settings. Although our study does not demonstrate an exact causal relationship, it indicates significant correlations between measured parameters and related symptoms.
Limitations of the study
The cross-sectional nature of this study and the related temporal effect weaken the observed relationship between metal exposure and disease. Furthermore, workplace air measurements were not performed due to a lack of healthy results from the related units of the workplaces in the control group. Furthermore, smokers and alcohol drinkers were excluded from this study to eliminate the confounding effects of these factors. However, this may have led to selection bias due to incorrect self-reporting about these parameters.
This study was supported by the Yozgat Bozok University Scientific Research Foundation (Decision No: 6602c-TF/17-104).
- Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC (2009) Inflammation mediates varying effects in neurogenesis: relevance to pathogenesis of brain injury and neurodegenerative disorder. J Neurochem 108: 1343-1359. Link: http://bit.ly/2QyVH07
- Hickey WF (1999) Leukocyte traffic in the central nervous system: the participants and their roles. Semin Immunol 11: 125-137. Link: http://bit.ly/2T2VzaQ
- Lossinsky AS, Shivers RR (2004) Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Review. Histol Histopathol 19: 535-564. Link: http://bit.ly/39PCyyu
- Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, et al. (1996) Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson's disease. Neurosci Lett 211: 13-16. Link: http://bit.ly/2FxQKhY
- Scalzo P, Kümmer A, Cardoso F, Teixeira AL (2009) Increased serum levels of soluble tumor necrosis factor-alpha receptor-1 in patients with Parkinson's disease. J Neuroimmunol 216: 122-125. Link: http://bit.ly/2QyeOau
- Fan Z, Aman Y, Ahmed I, Chetelat G, Landeau B, et al. (2015) Influence of microglial activation on neuronal function in Alzheimer's and Parkinson's diseasedementia. Alzheimers Dement 11: 608-621. Link: http://bit.ly/2N3Uzj6
- Li D, Song X, Huang H, Huang H, Ye Z (2018) Association of Parkinson's disease-related pain with plasma interleukin-1, interleukin-6, interleukin-10, and tumour necrosis factor-α. Neurosci Lett 683: 181-184. Link: http://bit.ly/35CfAYq
- Taylor A, Akdis M, Joss A, Akkoç T, Wenig R, et al. (2007) IL-10 inhibits CD28 and ICOS costimulations of T cells via src homology 2 domain-containingprotein tyrosine phosphatase 1. J Allergy Clin Immunol 120: 76-83. Link: http://bit.ly/2N7bFMT
- Chung F (2001) Anti-inflammatory cytokines in asthma and allergy: interleukin-10, interleukin-12, interferon-gamma. Mediators Inflamm 10: 51-59. Link: http://bit.ly/2umTNHc
- Hirano T, Taga T, Nakano N, Yasukawa K, Kashiwamura S, et al. (1985) Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2). Proc Natl Acad Sci USA 82: 5490-5494. Link: http://bit.ly/2T2XvQH
- Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol 8: 382-397. Link: http://bit.ly/39Q5DKk
- Dong Y, Dekens DW, Deyn PPD, Naudé PJW, Eisel LMU (2015) Targeting of Tumor Necrosis Factor Alpha Receptors as a Therapeutic Strategy for Neurodegenerative Disorders. Antibodies 4: 369-408. Link: http://bit.ly/36Cnfrc
- Woodside DG, Kram RM, Mitchell JS, Belsom T, Billard MJ, et al. (2006) Contrasting roles for domain 4 of VCAM-1 in the regulation of cell adhesion and soluble VCAM-1binding to integrin alpha4beta1. J Immunol 176: 5041-5049. Link: http://bit.ly/2QV1trY
- Huang J, Upadhyay UM, Tamargo RJ (2006) Inflammation in stroke and focal cerebral ischemia. Surg Neurol 66: 232-245. Link: http://bit.ly/35Bejke
- Richard S, Lagerstedt L, Burkhard PR, Debouverie M, Turck N, et al. (2015) E-selectin and vascular cell adhesion molecule-1 as biomarkers of 3-month outcome in cerebrovascular diseases. J Inflamm (Lond) 4: 12-61. Link: http://bit.ly/2FwbnuO
- Castillo J, Alvarez-Sabín J, Martínez-Vila E, Montaner J, Sobrino T, et al. (2009) Inflammation markers and prediction of post-stroke vascular disease recurrence: the MITICO study. J Neurol 256: 217-224. Link: http://bit.ly/35v9vx3
- Morello M, Zatta P, Zambenedetti P, Martorana A, D'Angelo V, et al. (2007) Manganese intoxication decreases the expression of manganoproteins in the rat basal ganglia: an immunohistochemical study. Brain Res Bull 74: 406-415. Link: http://bit.ly/39Opo4X
- Hassel B, Bachelard H, Jones P, Fonnum F, Sonnewald U (1997) Trafficking of amino acids between neurons and glia in vivo. Effects of inhibition of glialmetabolism by fluoroacetate. J Cereb Blood Flow Metab 17: 1230-1238. Link: http://bit.ly/2T63owu
- Michalke B, Fernsebner K (2014) New insights into manganese toxicity and speciation. J Trace Elem Med Biol 28: 106-116. Link: http://bit.ly/35zh45V
- Crossgrove J, Zheng W (2004) Manganese toxicity upon overexposure. NMR Biomed 17: 544-553. Link: http://bit.ly/302A37E
- Water E, Proal E, Wang V, Medina SM, Schnaas L, et al. (2018) Prenatal manganese exposure and intrinsic functional connectivity of emotional brain areas in children. Neurotoxicol 64: 85-93. Link: http://bit.ly/37G62Ns
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W (1994) Manganism and idiopathic parkinsonism: similarities and differences. Neurology 44: 1583-1586. Link: http://bit.ly/2QwPP7E
- Yang H, Wang J, Yang X, Wu F, Qi Z, et al. (2018) Occupational manganese exposure, reproductive hormones, and semen quality in male workers: A cross-sectional study. Toxicol Ind Health 35: 53-62. Link: http://bit.ly/2QwRkmj
- Ou SY, Luo HL, Mailman RB, Li ZC, Zhang WY, et al. (2018) Effect of manganese on neural endocrine hormones in serum of welders and smelters. J Trace Elem Med Biol 50: 1-7. Link: http://bit.ly/2QwRB8P
- Canfield RL, Henderson CR, Cory-Slechta DA, Cox C, Jusko TA, et al. (2003) Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med 348: 1517-1526. Link: http://bit.ly/37WGhc7
- Shobha N, Taly AB, Sinha S, Thuppil V (2009) Radial neuropathy due to occupational lead exposure: Phenotypic and electrophysiologicalcharacteristics of five patients. Ann Indian Acad Neurol 2: 111-115. Link: http://bit.ly/35C4SkC
- Tsai SY, Chou HY, The HW, Chen CM, Chen CJ (2003) The effects of chronic arsenic exposure from drinking water on the neurobehavioral developmentin adolescence. Neurotoxicology 24: 747-753. Link: http://bit.ly/39TBdXM
- Struzynska L, Dabrowska-Bouta B, Koza K, Sulkowski G (2007) Inflammation-like glial response in lead-exposed immature rat brain. Toxicol Sci 95: 156-162. Link: http://bit.ly/36CvdAC
- Kasten-Jolly J, Pabello N, Bolivar VJ, Lawrence DA (2012) Developmental lead effects on behavior and brain gene expression in male and female BALB/cAnNTac mice. Neurotoxicology 33: 1005-1020. Link: http://bit.ly/301gvjQ
- Liu X, Zheng G, Wu Y, Shen X, Jing J, et al. (2013) Lead exposure results in hearing loss and disruption of the cochlear blood-labyrinth barrier and the protective role of iron supplement. Neurotoxicology 39: 173-181. Link: http://bit.ly/2N5UStA
- Taheri L, Sadeghi M, Sanei H, Rabiei K, Arabzadeh S, et al. (2014) The relation between occupational exposure to lead and blood pressure among employed normotensive men. J Res Med Sci 19: 490-494. Link: http://bit.ly/2QVRhj1
- Kosnett MJ, Wedeen RP, Rothenberg SJ, Hipkins KL, Materna BL, et al. (2007) Recommendations for medical management of adult lead exposure. Environ Health Perspect 115: 463-471. Link: http://bit.ly/2QA75Jh
- Centers for Disease Control and Prevention (CDC) (2018) USA: Fourth National Report on Human Exposure to Environmental Chemicals Updated Tables. 1. Link: http://bit.ly/2Qxs7Iu
- Aliyev V, Kaya D, Yılmaz H, Soylemezoglu T (2012) The Potential Health Risk of Arsenic Levels in Workers Exposed to Arsenic. 48thCongress of the European Societies of Toxicology, Stockholm, Sweden. Toxicol Lett 102. Link: http://bit.ly/305QYWP
- McAdams RM, Juul SE (2012) The Role of Cytokines and Inflammatory Cells in Perinatal Brain Injury. Neurol Res Int 2012: 15. Link: http://bit.ly/2R0lH3H
- McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology 38: 1285-1291. Link: http://bit.ly/2R1af7E
- Liu B, Hong JS (2005) Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther 304: 1-7. Link: http://bit.ly/2sZc8tx
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Worley PF, Barnes CA, et al. (2005) Neuroinflammation alters the hippocampal pattern of behaviorally induced Arc expression. J Neurosci 25: 723-731. Link: http://bit.ly/39MaCvw
- Qian L, Block ML, Wei JS, Lin FC, Reece J, et al. (2006) Interleukin-10 Protects Lipopolysaccharide-Induced Neurotoxicity in Primary Midbrain Cultures by Inhibiting the Function of NADPH Oxidase. J Pharmacol Exp Ther 319: 44-52. Link: http://bit.ly/36LSSOM
- Lu CS, Huang CC, Chu NS, Calne DB (1994) Levodopa failure in chronic manganism. Neurology 44: 1600-1602. Link: http://bit.ly/2R30t5g
- Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, et al. (2006) Manganese exposure: neuropsychological and neurological symptoms and effects in welders. Neurotoxicology 27: 315-326. Link: http://bit.ly/2T4xpg0
- Guilarte TR (2010) Manganese and Parkinson’s disease: a critical review and new findings. Environ Health Perspect 118: 1071-1080. Link: http://bit.ly/2FwlzDA
- Tjalkens RB, Popichak KA, Kirkley KA (2017) Inflammatory Activation of Microglia and Astrocytes in Manganese Neurotoxicity. Adv Neurobiol 18: 159-181. Link: http://bit.ly/39OzdQn
- Kirkley KS, Popichak KA, Afzali MF, Legare ME, Tjalkens RB (2017) Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. J Neuroinflammation 14: 99. Link: http://bit.ly/2QB8X4c
- Litteljohn D, Mangano E, Clarke M, Bobyn J, Moloney K, et al. (2010) Inflammatory mechanisms of neurodegeneration in toxin-based models of Parkinson’s Disease. Parkinson’s Dis 2011: 713517. Link: http://bit.ly/37QtN5H
- Mokgobu MI, Cholo MC, Anderson R, Steel HC, Motheo MP, et al. (2015) Oxidative induction of pro-inflammatory cytokine formation by human monocyte-derived macrophages following exposure to manganese in vitro. J Immunotoxicol 12: 98-10. Link: http://bit.ly/35yjQbm
- Scharrer E, Hessel H, Kronseder A, Guth W, Rolinski B, et al. (2007) Heart rate variability, hemostatic and acute inXammatory blood parameters in healthy adults after short-term exposure to welding fume. Int Arch Occup Environ Health 80: 265-272. Link: http://bit.ly/39Q9qHt
- Yin L, Dai Q, Jiang P, Zhu L, Dai H, et al. (2017) Manganese exposure facilitates microglial JAK2-STAT3 signaling and consequent secretion of TNF-a and IL-1b to promote neuronal death. Neurotoxicology 64: 195-203. Link: http://bit.ly/2Fy4Bou
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
