International Journal of Immunotherapy and Cancer Research
Immunomodulatory Effects of Food Additives
Hamid Y Dar1, Shivani Chaturvedi1, Karishma Srivastava1, Zaffar Azam1, Rajaneesh Anupam1, Rajesh K Mondal1, Geetanjali B Tomar2, Ashish K Singh3, Pradyumna K Mishra4 and Rupesh K Srivastava1*
2Institute of Bioinformatics & Biotechnology, Savitribai Phule Pune University, Pune (MH)-411007, India
3School of Biochemical Engineering, Indian Institute of Technology-BHU, Varanasi (UP)-221005, India
4Department of Molecular Biology, National Institute for Research in Environmental Health, Bhopal (MP)-462001, India
Cite this as
Dar HY, Shivani C, Karishma S, Azam Z, Anupam R, et al. (2017) Immunomodulatory Effects of Food Additives. Int J Immunother Cancer Res 3(1): 019-031. DOI: 10.17352/2455-8591.000015Introduction
Food items that are unprocessed and do not contain preservatives, artificial colours, chemicals, fillers, artificial flavours etc are called as Natural foods. Natural foods are the best source of nutrition and health. Substances added to natural food to preserve flavour and increase their life are named as food additives. When food is to be stored for a long period, additives and preservatives are required to maintain the quality and flavour of the food items. The additives and preservatives prevent bacterial and fungal growth due to excess water in the foods [1]. Additives are defined by the United States Food and Drug Administration (FDA) as “any substance, the intended use of which results or may reasonably be expected to result, directly or indirectly, in its becoming a component or otherwise affecting the characteristics of any food.” Direct additives are those that are intentionally added to foods for a specific purpose. Indirect additives are those to which the food is exposed during processing, packaging, or storing. Preservatives are additives that inhibit the growth of bacteria, yeasts, and moulds in foods [2].
The drastic changes in agricultural and industrial practices over the past decades have increased the world’s capacity to provide food through increased productivity and diversity, decreased seasonal dependency and seasonal prices [3]. Increased consumption has been facilitated by rising income, urbanization, food industry marketing, media advertisement and trade liberalization, mainly in developed countries. Major shifts in dietary patterns are continually occurring, even in basic staples, consumption towards a more diversified and industrially processed food products. Living in westernized countries has a strong impact on nutritional patterns collectively termed as “Western diet” including high fat, trans fatty acids, cholesterol, proteins, sugars, salt intake, as well as frequent consumption of processed and “fast food” [4]. Influenced by this reality, populations of developing countries are undergoing a rapid change towards “transition nutrition”. Thus the traditional dietary pattern is gradually being replaced by the Western one [5].
Additives are used to provide a smooth and consistent texture, preserve the nutrient value and reduce the spoilage of food. Additives also control the acid-base balance of foods. Preservatives slow the process of product spoilage caused by mould, air, bacteria, fungi or yeast. Food additives are of great help in various techniques employed for food preparation and preservation, such as pickling, salting, and smoking, which were developed to deal with the emerging problems of storage, waste, and food-borne illnesses. The effects of food additives may be immediate or may be harmful in the long run on constant exposure. Sometimes immediate effects may also include headaches, change in energy levels and alteration in mental concentration, behaviour and immune response. Long-term effects of food additives had also been linked to increased risk of cancer. Some widely used food additives are Sodium benzoate (E211), Tartrazine (E102), Quinoline yellow (E104), Sunset yellow (E110), Carmosine (E122) and Allura red (E129) to name a few. Depending on the test protocol followed, it has been found that between 10-40% of aspirin-sensitive patients are indeed usually also affected by tartrazine, the reactions include asthma, urticaria, and rhinitis and childhood hyperactivity [6]. Sunset Yellow (E110), used in biscuits, has been found to damage kidneys and adrenals when fed to laboratory rats [7]. Benzoates (E210-E219), used mainly in marinated fish, fruit-based fillings, jam, salad cream, soft drinks and beer have been found to provoke urticaria, angioedema and asthma [7]. Sulphites (E220-E227) fed to animals have also been found to have mutagenic action [7]. Monosodium glutamate (MSG) a flavour enhancer, used in savoury foods, snacks, soups, sauces and meat products, has been associated with a conjunction of symptoms in susceptible individuals, such as severe chest and/or facial pressure and overall burning sensations, not unlike a feeling that the victim is experiencing a heart attack [6]. Consumption of artificial sweeteners has also been linked to various behavioural problems, hyperactivity, allergies and possibly carcinogenesis. Aspartame, sodium cyclamate and saccharine are used as artificial sweeteners with low calorie in fruit juices, jam & jelly. Corn starch, waxes and gums are used as food stabilizers. Incidental additives is the term applied to any substances that come into contact with food during its growth, processing or packaging. Intentional additives are those substances of known composition that are added to food to serve some useful purpose [8]. The FDA maintains a list of over 3000 ingredients in its database “Everything Added to Food in the United States”, many of which we use at home every day (e.g., sugar, baking soda, salt, vanilla, yeast, spices and colours, table 1. Food additives added to food for a specific purpose for example xanthan gum - used in salad dressings, chocolate milk, bakery fillings, puddings and other foods to add texture are called as direct food additives. Most direct additives are identified on the ingredient label of foods [12]. Food additives that become part of the food in trace amounts due to its packaging, storage or other handling, for instance minute amounts of packaging substances which find their way into foods during storage are called as indirect food additives. Food packaging manufacturers must prove to the U.S. FDA that all materials coming in contact with food are safe before they are permitted for use in such a manner [9].
Types of food additives
Preservatives: Food preservatives prevent the growth of microbes or spoilage and help in preserving flavour, texture, edibility and nutritive value of the food. Natural food preservatives come in the form of salt, sugar, alcohol, vinegar etc. These are the traditional preservatives in food that are also used at home while making pickles, jams, juices etc. Sugar and salt are the earliest natural food preservatives that very efficiently prevent the growth of bacteria in food [9]. To preserve meat and fish, salt is still used as a natural food preservative. During the past few decades the use of chemical food additives has increased tremendously. They seem to be the best and most effective for longer shelf life and are generally fool proof for preservation purposes [9]. Examples of chemical food preservatives are: Sodium Nitrate (251), Benzoic acid (210), Sodium Benzoate (211) and Sodium Sulphite (221). Artificial preservatives are the chemical substances that stop or delay the growth of bacteria thereby preventing spoilage and its discoloration. These artificial preservatives can either be added or sprayed on the food [9].
Sweeteners: Sweeteners provide sweet taste similar to that of sugar with or without any extra calories. Caloric sweeteners. Artificial sweeteners are synthetic sugar substitutes, but may be derived from naturally occurring substances, including herbs or sugar itself. Artificial sweeteners are also known as intense sweeteners because they are many times sweeter than sugar but contribute only few calories when added to foods [9] such as; Acesulfame-K, Sucralose, Sorbitol (420), Alitame (956), Aspartame (951), Saccharin/calcium saccharin etc (954). A high intensity sweetener is regulated as a food additive, unless its use as a sweetener is generally recognized as safe (GRAS). They do not contribute calories or only contribute a few calories to the diet and thus do not raise blood sugar levels [14]. High-intensity sweeteners are widely used in foods and beverages marketed as “sugar-free” or “diet,” including baked goods, soft drinks, powdered drink mixes, candy, puddings, canned foods, jams, jellies, dairy products, and scores of other foods and beverages [9].
Colour additives
A colour additive is any dye, pigment or substance that can impart colour alone or through reaction with other substances, when added to a food, drug or cosmetic applied to the human body. Any substance not normally consumed as a food in itself and not normally used as a characteristic ingredient of food, whether or not it has nutritive value [9]. Food colours are food additives which are added to make up for colour losses following exposure to light, air, moisture and variations in temperature and enhance naturally occurring colours [10]. Food colours are present in many foods including snack foods, margarine, cheese, jams and jellies, desserts, drinks, etc. with the following colour additives viz. Curcumin (110), Brilliant blue FCF (133), Tartrazine (102), Sunset Yellow FCF, Orange Yellow S.
Flavour enhancers
Flavour enhancers are used in savoury foods to enhance the existing flavour in the food. They themselves do not have any flavour but enhance the flavour of food products [10]. Natural flavour enhancers increase the stability of food. Salt is commonly used as a natural flavour enhancer for food products [10]. Monosodium Glutamate (E621), Calcium Glutamate (623), Disodium 5′-ribonucleotides (635), Ethyl Maltol (637), Monosodium Glutamate (E621), Monopotassium Glutamate (E623), Calcium Diglutamate (626), Guanylic acid (E627), Sodium Guanylate (E630), Inosinic acid are most commonly used flavour enhancers. Flavour enhancers are also used in a wide range of foods including savoury snacks, prepared meals and condiments. Monosodium glutamate is the sodium salt of the naturally occurring amino acid glutamic acid. It has been used as a seasoning or flavour enhancer since it was first isolated from seaweed more than a century ago and is now recognised as the most pure example of savoury taste [10].
Anti-caking agents
Anti-caking agents are a type of food additive that are added to keep ingredients from clumping together after being packaged [8]. Anti-caking agents act either to absorb moisture or act as a sealant and repel water and oil [11]. Anti-caking agents reduce the stickiness of the chunked, diced, or shredded cheese and improves the functionality of cheese, in formulated of fine mesh vegetable flour, bentonite, cellulose, and antimycotic agents or bacterial cultures. Anti-caking agents also reduce the growth of yeasts and moulds. This property of anti-caking agents has now been exploited for use in various flavors, colors, enzymes and other supplements [11]. Bentonite (558), Calcium aluminum silicate (556),Calcium silicate (552), Sodium ferrocyanide, Decahydrate, Propylene glycol, Magnesium silicate, Magnesium oxide, Cellulose, Calcium silicate, Silicon dioxide are the most commonly used anti-caking agents.
Emulsifiers
When water and oil are mixed together and vigorously shaken, oil droplets disperse in water and upon stopping of shaking, the phases start to separate. However, when an emulsifier is added to the system, the droplets remain dispersed, and a stable emulsion is obtained. The emulsifier may be an aerating agent, starch complexing agent and/or crystallisation inhibitor [12]. Nature is good at making emulsions, and the classic example is milk, where a complex mixture of fat droplet is suspended in an aqueous solution. Lecithins (E322) are mixtures of phospholipids such as phosphatidyl choline and phosphatidyl ethanolamine, and are usually extracted from sources such as egg yolk and soybeans. The precise composition of the phospholipids depends on the source. They are used for salad dressings, baked goods and in chocolates [12]. Lecithin (322), Sorbitan Monostearate (491), Ammonium salt of Phosphatidic acids (442), PolyGlycerol Ester (PGE), Sorbitan Ester (SOE), PG Ester (PGME), CSL Calcium stearoyl di Laciate are used extensively as emulsifiers in various food items.
pH controlling agents
The pH is the negative logarithm of the hydrogen-ion concentration in aqueous solution. The pH of a food is the measure of that product’s acidity or alkalinity which maintains flavour of food. The acid ingredients maintain a constant acid level by lowering the pH and thus preserve foods by inhibiting microbial growth. Natural acids include acetic acid or vinegar and citric acid from citrus, malic acid and tartaric acid (a weak acid) [13]. Commonly used acidulants are Lactic acid (E270), Malic acid (E296), Phosphoric acid (E338) Acetic acid (260), Citric acid (330), Fumaric acid (297). Alkaline compounds such as Potassium citrate, Calcium carbonate, Calcium acetate, Sodium bicarbonate and Sodium lactates are also used to neutralize excess acids that otherwise produce unwelcome flavors.
Antioxidant
Antioxidants prevent foods from oxidising or going rancid. Oxidation is a real problem for food products which causes raw apples and potatoes to go brown. Antioxidants are used as food additives to preserve food for a longer period of time. They act as oxygen scavengers, as the presence of oxygen in the food helps the bacteria to grow that can ultimately harm the food [14]. Antioxidants are classified into two broad divisions, depending on whether they are soluble in water (hydrophilic) or in lipids (hydrophobic). In general, water-soluble antioxidants react with oxidants in the cell cytosol and the blood plasma, while lipid-soluble antioxidants protect cell membranes from lipid peroxidation [15]. Different kind of antioxidants acts in different ways to delay or minimize the process of oxidation in food. BHT is another synthetic antioxidant. It works in the same way as butylated hydroxyanisole, but has caused controversy, as it has produced adverse effects in dogs. However, it also has anticancer effects [15]. It is used in margarine, oils, crisps and cheese. This antioxidant helps in preventing the reactions leading to the breakdown of fats [14]. Ascorbic acid (vitamin C) used in beers, cut fruits, dried potatoes and jams helps in preventing the discoloration of food by preventing the oxidation and also act as a substitute of vitamin C in potatoes that is lost during processing [14]. Some commonly used antioxidants are; Butylated hydroxyanisole (320), Ascorbyl palmitate (304), Calcium ascorbate (302), Ascorbic acid (vitamin C), Selenium, Vitamin A, Beta carotene, hydrogen peroxide (H2O2), Hypochlorous acid (HClO).
Immune system
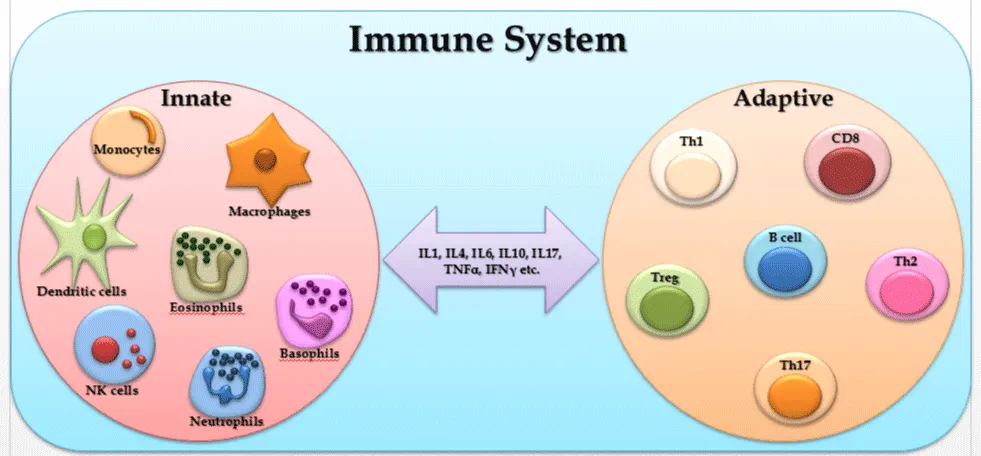
The immune system is a remarkably versatile defense system which has evolved for protection of animals from invading pathogenic microorganisms and cancer. It has ability to generate an enormous variety of cells and molecules which are capable of specifically recognizing and eliminating an apparently limitless variety of foreign invaders. A specific immune response giving rise to production of antibodies or T cells against a particular pathogen, known as adaptive immunity represents an adaptation that occurs during the lifetime of an individual as a result of exposure to that pathogen. Adaptive immune responses takes into account the clonal expansion of T and B lymphocytes bearing a huge repertoire of somatically generated receptors that can be selected to recognize virtually any pathogen. The adaptive immune system gets profoundly molded by the immunologic challenges encountered by that individual during the course of a lifetime. A great property of adaptive immune responses is that they being highly specific for the triggering agent and for this they provide the basis for immunologic memory. This unique property of memory endows the adaptive immune response with its “anticipatory” property, thus increasing resistance against future infection with the same pathogen and also allows vaccination against future infectious threats [15].
Adaptive immunity is important for the survival of all mammals and most other vertebrates, but different types of other mechanisms not involving antigen-specific lymphocyte responses are also involved in successful immune protection. These varied mechanisms are collectively known as innate immunity, since they are independent of prior exposure to specific pathogens for their amplification. These responses are controlled by the products of germline genes that are inherited and similarly expressed by all normal individuals. Innate immune mechanisms involve both constitutive and inducible components and use a wide variety of recognition and effector mechanisms. It has become clear in recent years that innate immune responses have a profound influence on the generation and outcome of adaptive immune responses via secretion of various cytokines. This ability of the innate immune system to instruct the responses of the adaptive immune system suggests many ways in which innate immunity can influence the development of both long-term specific immunity and autoimmune disease [15].
Recently over the last few years the impact of the gut microbiota on immune homeostasis has gained tremendous research interest. Also the gut harbors 60-80% of the host immune system. Thus the intestinal microbiome functions as a signaling hub that integrates environmental inputs, such as diet, genetic and immune signals to affect the host’s metabolism, immunity and response to infection. Host-microbial homeostasis involves appropriate immune regulation within the gut mucosa to maintain a healthy gut, while preventing uncontrolled immune responses against the beneficial commensal microbiota potentially leading to various inflammatory conditions such as chron’s disease, inflammatory bowel diseases (IBD) etc. This complex, bilateral interaction between the host and its microbiota has a crucial role in human health. Many ‘multifactorial’ disorders, formerly considered to be idiopathic, might therefore be influenced or even driven by alteration of the intimate crosstalk that occurs between the host immune system and the gut microbiota during homeostasis [16].
T cells
T lymphocyte development constantly confronts the dilemma of combating infection without provoking a response to the host. The price for generating an increasingly varied population of antigen receptors needed to recognize a wide spectrum of pathogens is the progressive risk of producing self-reactive lymphocytes that can provoke an autoimmune diathesis. To minimize the possibility of self-reactive cells, T lymphocytes are subjected to a rigorous selection process during development in the thymus. In addition, premature activation of mature T cells is prevented by requiring two signals for activation. Finally, the tremendous expansion of T cells that occurs during the response to an infection is resolved by the active induction of cell death. The consequences of inefficient lymphocyte removal at any one of these junctures can be devastating to the health of the organism [17].
Th1 cells: Th1 cells, are characterized by the secretion of Interferon-gamma (IFN-γ) (Table 2) and tumour necrosis factor alpha (TNF) [18]. Th1 cells are responsible for cell-mediated immune responses and an excessive Th1 response will result in tissue damage [19]. Th1 cells are most often defined by their production of IL-2 and IFN-γ but have been reported to produce a number of cytokines including: TNF, lymphotoxin, and granulocyte-macro-phage-colony-stimulating factor (GM-CSF) [18]. The signature cytokine of the Th1 subset, IFN-γ, has long been associated with pathology of several autoimmune diseases including autoimmune type 1 diabetes (T1D), multiple sclerosis (MS) and rheumatoid arthritis (RA) [20,21] Th1 cells promote immune pathology in MS/EAE, conceivably by secreting IFN-γ, which plays an essential role in promoting autoimmune pathology [22]. IFN-γ signalling, but not Th2 cytokines, was found to be crucial for the generation and production of auto antibodies targeting intracellular molecules, similar to those found in SLE [23, 24] and IFN-γ production was found to be elevated in serum of patients with SLE [25]. These results highlighted the pathogenic role of IFN-γ in autoimmune diseases, even in a disease like SLE that was initially considered to be a Th2/type-2 mediated autoimmune disease (i.e. humoral-mediated) [18] (Figure 1).
Th2 cells: Th2 cells secrete IL-4, IL-5 and IL-13 [27]. Th2 are responsible for humoral-mediated immunity and excessive Th2 responses can result in atopy/ hypersensitivity [19]. Th2 cells are recognized for their role in host defense against multi-cellular parasites and their involvement in allergies and atopic illnesses [18]. Th2 differentiation and function are intimately regulated by innate and epithelial cell types that inhabit these tissues [26]. Th2 cells are best known for the production of IL-4, IL-5 and IL-13, as well as IL-9 and IL-10 [27]. IL-4 is a multifunctional, pleiotropic cytokine discovered in the early 1980s’, which is mainly produced by activated Th2 cells, but also by mast cells, basophils, eosinophils and T cells [28,29]. In experimental models of helminth infection, Th2 cells are thought to promote tissue repair by promoting the function of M2 macrophages through secretion of IL-4 [30]. In autoimmune diseases, Th2 cells were initially described as anti-inflammatory based on their ability to suppress cell-mediated or Th1 models of disease [18,19]. Th2 cells are best known for the production of IL-4, IL-5 and IL-13, as well as IL-9 and IL-10 [21].
Th17 cells: Th17 cells differentiate from naive T cells in the presence of TGF-β plus inflammatory stimuli such as, IL-1β, IL-6, IL-21, and IL-23. IL-23 is dispensable for the lineage commitment of Th17 cells but is required for the growth, survival, and functions of Th17 cells [31,32]. During infection, IFN-γ regulates the induction and expansion of pathogenic Th17 cells [33]. These properties of IFN-γ seem to be pivotal in down regulating the inflammatory responses mediated by other Th cells and pathology promoted by these cells, in particularly Th2 and Th17 cells [34]. TGF-β is essential for the generation of both induced regulatory T cells (iTregs) and Th17 cells via the induction of FoxP3 and RORγt. However, in the absence of inflammation, FoxP3 represses RORγt and promotes iTregs. Signaling via inflammatory cytokines, such as IL-6, IL-21, and IL-23, results in STAT3 phosphorylation, relieves RORγt from the suppression of FoxP3, and initiates Th17 programming. STAT3 in combination with IFN regulatory factor 4 (IRF4) further induces RORγ expression. The transcription factors STAT3, RORγt, and Runx1 bind to the promoter regions of the IL-17, IL-21, IL-22, and CCL20 genes and induce IL-17, IL-21, IL-22, and CCL20. Th17 programming can be antagonized by cytokines, such as IFN-γ, IL-2, and IL-27. IL-2-mediated and IL-27-mediated activation of STAT5 and STAT1 inhibit STAT3, whereas T-bet induced by IFN-γ can block RORγt [35]. Compared with Th1, Th2, and natural Tregs, Th17 cells display instability. In mice and humans, Th17 cells co-expressing IL-17/IFN-y, RORyt/T-bet, or FoxP3/IL-17/RORyt have been observed during inflammatory responses [36]. IFN-γ can up-regulate IL-12/Roryt on Th17 cells and enhance their sensitivity to IL-12, resulting in a Th17/Th1 phenotype that stably co expresses RORγt and T-bet [37]. Th17 cells secrete several effector molecules, including IL-21, IL-22, IL-17A/F, and CCL20. These soluble factors act on both immune and non-immune cells and mediate several functions, such as differentiation of cells; release of antimicrobial molecules, cytokines, and chemokines; and recruitment of cells to sites of inflammation [37]. The prevalence of IL-17 and IL-22 CD4+ T cells is increased in the circulation of patients with RA and ankylosing spondylitis; these cells produce higher quantities of IL-17 after stimulation [38]. IL-17 is also present at the sites of inflammatory arthritis and amplifies the inflammation induced by other cytokines and, in particular, TNF-α. In a collagen-induced arthritis (CIA) model, the disease is mainly mediated by IL-17 because IL-17 deficiency, or treatment with IL-17RA antagonist or with IL-17-neutralizing antibody before disease onset, attenuates arthritis with decreased joint damage and reduced serum IL-6 [39].
Treg cells: Treg cells can be subdivided into naturally arising cells (nTreg) that are generated in the thymus, and inducible Treg (iTreg) that are converted into Treg upon activation in the periphery [40]. Natural Tregs are a population of CD4+ T lymphocytes residing in the thymus and constitute 5-10% of the peripheral naive CD4+ T lymphocyte pool in normal mice and humans [41]. Induced Tregs are found in peripheral lymphoid tissues from naive T cells [42]. These iTregs development is driven by the release of suppressor cytokines such as IL-10 and TGF- β [43]. The current notion is that nTreg cells mediate suppression in a cell contact-dependent manner, while iTreg cells predominantly mediate suppression via cytokine-dependent pathways by releasing suppressor cytokines such as TGF-β and IL-10 [44]. Treg cells may play a crucial role in human autoimmune diseases by exerting their suppressive function, and Treg-related somatic cell therapy is considered as an intriguing new intervention for autoimmune diseases [45]. Tissue homing is modulated prominently by lymphocytes, including regulatory T cells (Tregs) [46]. Tregs play a pivotal role in the maintenance of homeostasis between immune response and immune tolerance [47]. Although the majority of Treg appears within the CD4+ T cell set, suppressor activity was also reported among CD8+ T cells [48]. Over the last few years, however, most attention was focused on CD4+ regulatory cells and particularly the nTreg, which are characterized by constitutive expression of the α-chain of the IL-2 receptor (CD25) and the transcription factor Foxp3 [49]. Foxp3 is essential in the development and function of nTreg which is Foxp3 inhibits IL-2 transcription and induces up-regulation of Treg-associated molecules, such as CD25, CTLA-4 and GITR [50], that can down-regulate the immune response of adjacent cells. IL-10 can suppress differentiation of Th1 and Th2 cells directly by reducing IL-2, TNF-α and IL-5 production, and also indirectly by down-regulating MHC and co-stimulatory molecules on APC, thereby reducing T cell activation [48].
B-cell
B cells are lymphocytes that use B cell receptor molecule for recognition of antigens. The B cell receptor consists of a surface immunoglobulin molecule for recognition of the antigen, and two associated proteins for transduction of the signal. On encounter with its antigen, B cells initiates a process of activation leading to antibody secretion and memory formation regulated by interplay with antigen-activated T cells, dendritic cells, soluble factors, and in some cases follicular dendritic cells. Both T and B lymphocytes can differentiate from naïve to memory cells, but only B cells have the capacity to fine tune their antigen receptor structure to increase its specificity and affinity, giving rise to more effective antibodies. Beyond immunoglobulin secretion, B cells regulate the immune response by cytokine secretion and antigen presentation to T cells in the context of class II molecules.
B cell-mediated autoimmunity is the consequence of the production of self-reactive antibodies. There are multiple reported mechanisms operating throughout B cell maturation and differentiation that are designed to avoid auto reactivity. The failure of only one tolerance checkpoint rarely leads to autoimmune disease [51]; it may, however, increase the level of circulating auto antibodies, without clinical disease.
Dendritic cells
Dendritic cells (DCs) are highly potent antigen presenting cells (APCs) that play a key role in both the initiation and regulation of T cell mediated immunity to pathogens and tumors along with preventing immune responses against self-tissues. DCs are implicated in the induction of immunity and in the maintenance of tolerance. DCs represent a sparsely distributed population of bone marrow–derived mononuclear cells that are found in most tissues of the body. In the immature state, they are primed to capture antigens through expression of several receptors that enable recognition and acquisition of foreign and self-antigens [15]. Upon encountering pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs), DCs undergo a process of “maturation,” involving changes in the DC phenotype, antigen acquisition capacity, migration, and ability to traffic to draining lymph nodes, where they prime humoral and cellular immune responses [15]. Under steady state conditions, DCs play an active role in maintaining tolerance to self-antigens. Although most autoimmune cells are deleted in the thymus through a process of negative selection, others must be tolerized through active and sustained mechanisms.
Macrophages
Macrophages are mononuclear cells of the myeloid lineage, and are responsible for maintaining homeostasis and tissue repair. Macrophages play a significant part in immunity and immune responses. They assume a defensive role exhibited by their ability to carry on phagocytosis of parasites and microbes. They regulate lymphocyte activation and proliferation and are essential in the activation process of T and B lymphocytes by antigens and allogenic cells. Enhanced bactericidal activity of “activated macrophages” is based on immunologically linked mechanisms involving lymphocytes. Macrophages kill ingested microbes but the mechanism by which this is accomplished is not yet completely understood [52].
Eosinophil
Eosinophils are also called acidophils which are responsible for combating multicellular parasites and certain infections in vertebrates. These cells have a nucleus with two lobes (bilobed) and cytoplasm filled with large granules. These cells are implicated in inflammatory processes and allergic disorders. CCL11 (eotaxin-1) also augments bone marrow release of mature eosinophils and eosinophil precursors via engagement of CCR3 receptors, which are expressed mainly on eosinophils [53]. Eosinophil-derived neurotoxin (EDN) serves as an endogenous ligand of TLR2, can activate Myd88 in dendritic cells, and shifts adaptive immunity toward a Th2 response, suggesting a pivotal role for esoinophils in the innate-adaptive immune response [54]. Similar to neutrophils, eosinophils are able to generate extracellular traps with bactericidal properties, but they do not undergo apoptosis upon release of their DNA like Neutrophils. Eosinophils possess immunoglobulin (Ig) G receptors which help in activation of eosinophils.
Neutrophil
Neutrophils are also called ‘polymorphonuclear cells.’ These play very important roles in our innate immune system by first migrating to the site of the infection to begin killing of the invading microbes. Neutrophils have more than one nucleus and lobulated shape. They are making up about 60% of the immune cells and contain antimicrobial effectors. Granulocyte colony-stimulating factor (G-CSF) effects include induction of myeloid differentiation, proliferation of granulocyte precursors, and release of mature neutrophils from the marrow [55]. Neutrophil matrix metalloproteinases (MMPs) function is not limited to bacterial killing because MMPs are also important for extravasations and diapedesis [56]. Neutrophil life spans may be modulated by soluble signals: when exposed to stimuli such as TNF and Fas (CD95) ligand, neutrophils undergo apoptosis or programmed cell death [57,58].
Mast cell
Mast cells are “master regulators” of the immune system. They come from bone marrow and go into all tissues of the body. Mast cells contains secretory granules (storage sacs) which are biologically active molecules and released from cells when triggered, leading to allergic and inflammatory responses. Mast cells are among the first immune cells to encounter pathogens invading into tissue from the external world or via the bloodstream, consistent with their role as immune sentinel cells [59]. Mechanisms of reducing mast cell numbers include apoptosis, demonstrated in tissue mast cells deprived of the cytokine stem cell factor, a critical survival signal for mast cells [60,61]
Natural killer (NK) cells
NK cells are specialized to kill certain types of target cells, especially virus infected and cancerous cells. NK cells recognize potential targets which are not diversified between T cell receptors (TCRs) and B cell antigen receptors (BCRs). NK cells secrete cytokines such as anti-viral cytokine IFN-γ and inflammatory cytokine TNF-α to killing target cells. NK cells are crucial components of the innate immune system and, as the name suggests, they do not require pre-stimulation to perform their effector functions. Morphologically, they are characterized as large, granular, bone marrow-derived lymphocytes and phenotypically, they are defined as CD56+ CD3ε+ in humans. They represent 10% of the cells in the total peripheral blood mononuclear cell PBMCs population of circulating human lymphocytes and they comprise the third largest population of lymphocytes following B and T cells. They are also found in the peritoneal cavity, spleen, liver, lung, lymph nodes, thymus, and in uterus during gestation [62].
Effect of food additives on Immune system
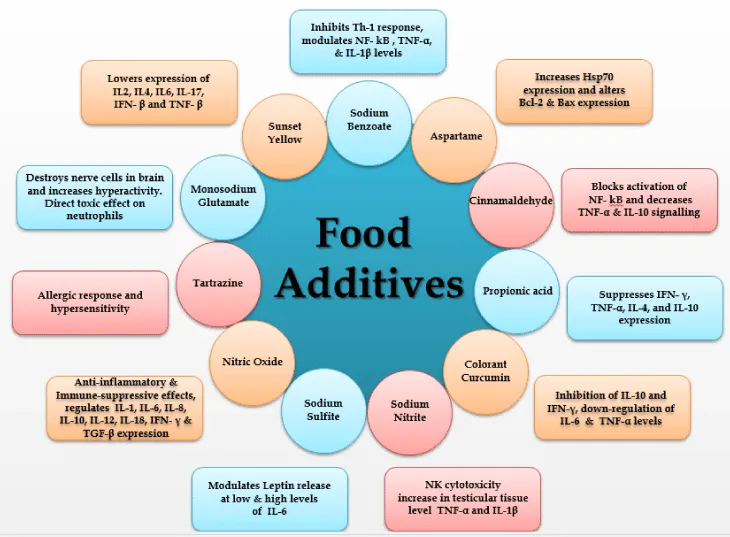
Sodium benzoate (NaB): Sodium benzoate, a metabolite of cinnamon and a FDA-approved drug against urea cycle disorders in children, is a widely used food additive, which is long known for its microbicidal effect [65] (Figure 2). Recent discoveries suggest that NaB is an important modulator of adaptive and innate immune responses of experimental allergic encephalomyelitis (EAE) leading to attenuation of inflammation and demyelination in EAE [66]. Sodium benzoate counteracts autoimmune diseases by decreasing inflammation via inhibition of T cell proliferation, the mevalonate pathway, iNOS, NF-κB, TNF-α, IL-1β, Th-1, and adhesion molecules and by increasing Tregs [66]. The release of the T cell-derived lymphokine leucocyte inhibitory factor (LIF), in response to incubation with sodium benzoate or other food additives and with acetylsalicylic acid (ASA), was measured in vitro [67]. Sodium benzoate has ability to deprive cells of oxygen, break down the immune system and cause cancer. Sodium benzoate chokes out our body’s nutrients at the cellular level by depriving cells of oxygen, sometimes completely shutting them down. NaB did not affect the relative expression of CD3e or CD4 molecules following 72 h exposure, however, it down regulated the relative expression of CD8 T cell co-receptor and lowered the expression of IL4, IL6, IFN- γ and IL17 cytokines in Con A stimulated splenocytes; and IL6, IFNγ and TNF-α in LPS stimulated splenocytes following 48 h of exposure [68]. Sodium benzoate can also regulate many immune signaling pathways responsible for inflammation, glial cell activation, switching of T-helper cells, modulation of regulatory T cells, cell-to-cell contact, and migration [65]. NaB induces the expression of TGF-β mRNA and protein in normal as well as proteolipid protein primed splenocytes and the presence of a consensus STAT6 binding site in the promoter of the TGF- β, gene, activation of STAT6 in splenocytes by NaB, recruitment of STAT6 to the TGF- β promoted by NaB, and abrogation of NaB-induced expression of TGF-β in splenocytes by small interfering RNA knockdown of STAT6 suggest that NaB induces the expression of TGF- β via activation of STAT6 [69]. Sodium benzoate (E211), the salt of benzoic acid, is a well characterised food preservative contained in food as well in cosmetic. Benzoic acid is also found naturally in apricots and other foods [70]. NaB on the other hand can also be used as a potential drug candidate for therapy of MS. [73]. Propionic acid (E280), sodium benzoate and colorant curcumin, all three tested compounds suppressed pathways linked with Th1-type immune activation, which indicates an anti-inflammatory property [70]. NaB inhibited the production of TNF-α and IL-1β protein in dose-dependent manner and the expression of TNF-α and IL-1β mRNA [71].
Aspartame
Aspartame, a “first generation sweetener”, is widely used in a variety of foods, beverages, and medicines. The FDA has determined the acceptable daily intake (ADI) value of aspartame to be 50 mg/kg per day, while the JECFA (Joint FAO/WHO Expert Committee on Food Additives) has set this value at 40 mg/kg of body weight/day. The immune system is now recognized as a target organ for many xenobiotics, such as drugs and chemicals, which are able to trigger unwanted apoptosis or to alter the regulation of apoptosis. It has been observed that oral administration of aspartame for 90 days did not cause any apparent DNA fragmentation in immune organs of aspartame treated animals; however, there was a significant increase in hsp70 expression, apart from significant alteration in bcl-2 and bax at both mRNA transcript and protein expression level in the immune organs of aspartame treated animals compared to controls. Hence, these results indicated that hsp70 levels increased in response to oxidative injury induced by aspartame metabolites; however, these metabolites did not induce apoptosis in the immune organs [72].
Cinnamaldehyde
Low concentrations (up to 1 mg/ml) of CA results in a slight increase in nuclear factor-κB activation, whereas higher concentrations led to a dose-dependent decrease of nuclear factor-κB activation (up to 50%) in lipopolysachharide-stimulated THP1 cells and PBMCs. Accordingly, nitric oxide, IL-10 secretions as well as cell proliferation were reduced in lipopolysachharide-stimulated RAW264.7 cells, PBMCs and THP1, Raji and Jurkat-E6 immune cells in the presence of CA in a concentration-dependent manner. Flow cytometric analysis of PBMCs revealed that CD3+ was more affected than CD20+, having the ability to block nuclear factor-kB activation in immune cells [82]. Treatment with CA led to inhibition of cell viability, proliferation and induced apoptosis in a dose-dependent manner in primary and immortalized immune cells. Its described anti-carcinogenic property in cancer patients might be contraindicated due to its ability to inhibit immune cell activation [73]. Cytokines (pro-or anti-inflammatory, TNF-α or IL10), of signaling molecules (NO) as well as pathways (NF-kB/AP-1) investigated, a decrease in activation and production was observed when using higher concentration of CA than 1 mg/ml ( = 8 mM) [82].
Propionic acid
Propionic acid (PA) suppresses IFN-γ-mediated neopterin production and Trp degradation in PBMCs in a dose-dependent manner. This suppressive effect on Th1-type immunity may be helpful in inflammatory conditions. It may also be harmful by diminishing the efficacy of the immune system to respond against pathogens and tumors and may even promote allergic diseases [70]. PA could inhibit the production of inflammatory (TNF-α) as well as anti-inflammatory (IL-4, IL-10) cytokines and also decreases the production of a number of chemokines (IL-8, MIP-1a and MIP-1b, CCL5 and CXCL10).
Colorant curcumin
Neopterin concentrations in culture supernatants decreased in a dose-dependent way by addition of curcumin to unstimulated PBMCs. Curcumin also suppressed Trp degradation in a dose-dependent manner: Trp concentrations increased and in parallel Kyn levels and thus kyn/Trp declined when compared to PBMCs stimulated with PAH, but not exposed to curcumin. Within the cellular immune response, pro-inflammatory cytokine IFN-β strongly induces enzyme GTP-cyclohydrolase-I which produces neopterin and IDO which converts Trp to Kyn in human macrophages and other target cells. Neopterin production and Trp degradation can be used to monitor Th1-type immune activation. Food additives curcumin, suppress IFN- β mediated neopterin production and Trp degradation in PBMC in a dose-dependent manner [70]. Curcumin decreased LPS-stimulated secretion of IL-6, and also affect the leptin release after co-incubation with LPS from cultured adipocytes in a dose- and time-dependent manner [81]. Curcumin supplementation also resulted in inhibition of LPS-induced IL-10 and IFN-γ and in stimulation of IL-4 secretion [73]. Long term effects of curcumin shows down-regulation of IL-6 and TNF-α production [73].
Sodium Nitrite
There are no significant differences in the WBC count of mice exposed to sodium nitrite. Significant decrease in the percentage of lymphocytes in mice when treated with 100 mg/kg-BW sodium nitrite along with significant increase in the percentage of neutrophil count has been reported. Sodium nitrite has a significant dose-dependent suppression of NK cytotoxicity on WEHI-1640 tumor cells. Sodium nitrite causes an inflammation that induces influx of a large number of cells, including neutrophils and macrophages that subsequently release large quantities of potential oxidants such as H2O2 that might induce damage to surrounding tissues and cells [75]. Sodium nitrite treatment results in significant increase in testicular tissue level of Malondialdehyde (MDA), TNF-α and IL-1β [76].
Sodium Sulfite (SS)
In the past 30 years, SS has become one of the leading food preservatives in the food sector throughout the world. The measured concentrations of SS in food vary between 0.8mM, i.e. in dried potatoes, and 1.6mM, i.e. in wine and dried fruits. In 1983, the Joint Expert Committee on Food Additives of the FAO of the WHO established an acceptable daily intake level of 0.7 mg/kg body weight. SS affect the leptin release after co-incubation with LPS from cultured adipocytes in a dose- and time-dependent manner. There is no direct effect on leptin secretion after incubation with antioxidants in the absence of LPS, at low levels of IL-6. However, at high levels of IL-6 due to LPS co-incubation, leptin secretion was significantly less than in the absence of antioxidants [71].
Nitric Oxide (NO)
The suppression of Foxp3 in MBP-primed T cells is due to the direct effect of NO signaling, whereas the suppression of CD25 is a secondary consequence of NO, which was evident from the inability of donor NO to decrease the population of CD25+ Foxp3+ cells in iNOS2 T cells . Reduction in the mRNA level of CD25 after MBP stimulation is probably due to the direct effect of down-regulation of Foxp3. This confirmed the inverse relationship between NO and Foxp3 by analyzing the mRNA expression of Foxp3 and characterizing CD25+ FoxP3+ or CD4+Foxp3+ phenotypes from inducible NO synthase knockout mice. NO inhibited the expression of Foxp3 in MBP-primed T cells via soluble guanylyl cyclase-mediated production of cGMP. Taken together, these results imply a novel role of NO in suppressing Foxp3+ Tregs via the soluble guanylyl cyclase pathway [77].Production of nitric oxide results in anti-inflammatory–immunosuppressive effect and thus inhibition of T cell proliferation, B cell proliferation and antibody production by CD25+ B cells and regulation of IL-1, IL-6, IL-8, IL-10, IL-12, IL-18, IFN- γ, TGF-β [78].
Tartrazine
It is also known as FD&C Yellow # 5. It provides yellow colour and can be found in green and blue candies. There is currently a petition to the FDA to ban tartrazine from food. Some schools have banned products containing tartrazine and subsequently noticed a big difference in the overall behaviour of their students. Tartrazine is a coal tar derivative, like most artificial colourings, and is one of the most controversial of the azo dies used in food. Norway has banned tartrazine because this chemical has been linked to severe allergic reactions, especially in asthmatics and is one of the food additives thought to be a cause of hyperactivity in children [79].
Monosodium Glutamate (E621)
It is a flavour enhancer additive which is used to bring out the flavour without adding a flavour of their Natural source. It can destroy nerve cells and linked with aggravating or accelerating Huntington’s, Alzheimer’s and Parkinson’s diseases. It may cause cancer, DNA damage and fetal abnormalities in animals and is also linked with increased hyperactivity [80]. MSG is a known Excitoxins (glutamate, aspartate, and cysteine) which kills brain cells through a mechanism which causes the cells to fire repeatedly until they self-destruct. MSG and aspartame, an artificial sweetener, are the most common excitotoxins which may causes headache, nausea, weakness, and burning sensation in the back of neck and forearms [79]. MSG has a direct toxic effect on the neutrophils in the blood or it has a deleterious effect on blood production in the bone marrow, especially on the progenitor cells (aplasia) and that it is time-dependent [81]. This might be indicative of the deterioration of immune status to the toxic effect of MSG.
Sunset yellow FCF
Sunset yellow FCF (SY), a permitted food color, is extensively used in various food preparations and quite often exceeds the permissible levels (100-200 mg/kg). It was observed that SY (250 μg/ml) significantly suppressed the mitogen induced proliferation of splenocytes and MLR response. Further, immunophenotypic analysis revealed that SY alters the relative expression of CD3e/CD4/CD8 in T cells and CD19 in B-cells. Consistent with the suppression of T-cell and B-cell responses and alters surface receptor expression, SY also lowers the expression of IL2, IL4, IL6, IL-17, IFN- β and TNF- β cytokines. These results suggest that non-cytotoxic dose of SY may have immunomodulatory effects [82].
Emulsifiers
The last half-century has witnessed enormous increase in the consumption of various food additives. Emulsifiers disturb the host–microbiota homeostasis resulting in enhanced mucolytic and pro-inflammatory activity thereby promoting intestinal inflammation such as colitis [83]. It is now well established that probiotics (a class of symbiotic bacteria whose administration in adequate amount provides health benefits to the host by altering the composition of gut microbiota) can enhance the gut microbiota and can help to overcome several health issues caused due by dysbiosis in the gut [84]. Carboxymethylcellulose (CMC), P80 and other emulsifiers currently used in food products impact human health in several ways. Studies by various groups in mice suggest the possibility that dietary emulsifiers contributed to the post-mid-twentieth-century increase in incidence of inflammatory bowel disease, metabolic syndrome, and perhaps other chronic inflammatory diseases [13].
Conclusion
The use of food additives has increased to a great extent in the last few decades. At present, it has been estimated that about 85% of the Western diet is made up of various processed foods. Presently on an average each person is consuming 8-10 lbs of food additives per year, with some possibly eating even more. The consumption of food additives has been linked with the increase of following disorders in animals: eczema, urticaria, angioedema, exfoliative dermatitis, irritable bowel syndrome, nausea, vomiting, diarrhoea, rhinitis, bronchospasm, migraine, anaphylaxis, hyperactivity, autoimmune diseases and other behavioural disorders. High concentrations of food additives are usually used in sweets, desserts, cereal bars, drinks, and almost all frozen manufactured foods. They are used to mask the poor quality of the ingredients in these foods. When people consume foods containing these additives in large amounts, they can experience toxic effects. The risk associated with the ever increasing use of copious amount of food additives in our daily life is posing a huge risk to our immune system leading to various disorders and diseases. Thus future research on this very intricate and important aspect of the link between nutrition and immune system would not only reveal the molecular mechanisms associated with this but would also lead to discovery of various therapeutics to revert these ill effects along with paving the path for various healthy options as food additives.
- (2010) Food ingredients & colors. International Food Information Council (IFIC) Foundation US Food and Drug Administration (FDA). Link: https://goo.gl/t4DZRf
- Branen AL, Davidson PM, Dekker M (2002) Second Edition Revised and Expanded. Link: https://goo.gl/re0krs
- Kearney J (2010) Food consumption trends and drivers. Philosophical Transactions of the Royal Society B: Biological Sciences 365: 2793–2807. Link: https://goo.gl/KWjM5i
- Manzel A, Muller DN, Hafler DA, Kleinewietfeld M, Erdman SE, et al. (2014) Role of ‘ Western Diet ’ in Inflammatory Autoimmune Diseases. Link: https://goo.gl/Eis34w
- Pereira Ra, Duffey KJ, Sichieri R, Popkin BM (2014) Sources of excessive saturated fat, trans fat and sugar consumption in Brazil: an analysis of the first Brazilian nationwide individual dietary survey. Public health nutrition 17: 113–121. Link: https://goo.gl/gSy2wP
- Tuormaa TE (1994) The Adverse Effects of Food Additives on Health : A Review of the Literature with Special Emphasis on Childhood Hyperactivity. Journal of Orthomolecular Medicine 9: 225–243. Link: https://goo.gl/F1tS1z
- Miller M (1985) Danger! Additives at Work, London Food Commission, London. Link: https://goo.gl/KFxB3L
- Belly Bytes-Types of Food Additives. Link: https://goo.gl/5mquW5
- (2010) International Food Information Council Foundation & US Food and Drug Administration. Food Ingredients & Colors. U.S Food and Drug Administration 1–8. Link: https://goo.gl/KvObqL
- (2016) efsa European food safety authority. Link: https://goo.gl/FNbOjZ
- Mirza SK, Asema UK, Kasim SS (2017) To Study The Harmful Effects Of Food Preservatives On Human Health. 610–616. Link: https://goo.gl/FY1bhg
- Chassaing B, Van de Wiele T, De Bodt J, Marzorati M, Gewirtz AT (2017) Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Link: https://goo.gl/73lYiL
- Budd RC, Fortner KA, Hughes PD, Belz GT, Strasser A, et al. (2013) Lymphocytes. Chapter 13: 15-190. Link: https://goo.gl/O0B57M
- McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, et al. (2000) Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood 95: 3489–3497. Link: https://goo.gl/KPfW6j
- Steven A, Porcelli T, Gary Firestein, Ralph Budd, Sherine E, et al. (2013) Lymphocytes Kelley’s textbook of Rheumatology, 9th edition, Chapter 18:255-267. Link: https://goo.gl/Kytzru
- Sommer F, Bäckhed F (2013) The gut microbiota-masters of host development and physiology. Nat Rev Microbiol 11: 227-238 Link: https://goo.gl/XklbtL
- Ralph Budd C, Karen A, Fortner T (2013) Lymphocytes Kelley’s textbook of Rheumatology, 9th edition. Chapter 13 174-190.
- Itay Raphaela, Saisha Nalawadea, Todd N, Eagarb, Thomas G, et al. (2015) "T cellsubsets and their signature cytokines in autoimmune and inflammatory diseases". Cytokine 74: 5-17. Link: https://goo.gl/JYV8OD
- Berger A ((2000) Science commentary: Th1 and Th2 responses: what are they? British Medical Journal 321, 424–424. Link: https://goo.gl/DOktbP
- Raphael I, Forsthuber TG (2012) Stability of T-cell lineages in autoimmune diseases. Expert review of clinical immunology 8: 299–301. Link: https://goo.gl/Rfegmo
- Skurkovich S, Skurkovich B (2005) Anticytokine therapy, especially anti-interferon-γ, as a pathogenetic treatment in TH-1 autoimmune diseases. Ann N Y Acad Sci 1051: 684–700. Link: https://goo.gl/H6mV5d
- Olsson T (1995) Critical Influences of the Cytokine Orchestration on the Outcome of Myelin Antigen-Specific T-Cell Autoimmunity in Experimental Autoimmune Encephalomyelitis and Multiple-Sclerosis. Immunological Reviews 144: 245–268. Link: https://goo.gl/WuIS0U
- Haas C, Ryffel B, Le Hir M (1998) IFN-gamma receptor deletion prevents autoantibody production and glomerulonephritis in lupus-prone (NZB x NZW) F1 mice. The Journal of Immunology 160: 3713–3718. Link: https://goo.gl/IrXjFZ
- Pollard KM, Hultman P, Kono DH (2005) Immunology and genetics of induced systemic autoimmunity. Autoimmunity Reviews 4: 282–288. Link: https://goo.gl/9fMIgO
- Preble OT, Black RJ, Friedman RM, Klippel JH, Vilcek J (1982) Systemic lupus erythematosus: presence in human serum of an unusual acid-labile leukocyte interferon. Science 216: 429–431. Link: https://goo.gl/Mq1aW8
- Gómez D, Correa PA, Gómez LM, Cadena J, Molina JF, et al. (2004) Th1/Th2 cytokines in patients with systemic lupus erythematosus: is tumor necrosis factoral phaprotectiv. Semin Arthritis Rheum 33: 404-413. Link: https://goo.gl/eDQOSt
- Vahedi G, Poholek CA, Hand TW, Laurence A, Kanno Y, et al. (2013) Helper T-cell identity and evolution of differential transcriptomes and epigenomes. Immunological Reviews 252: 24–40. Link: https://goo.gl/SQDOa7
- Howard M (1983) Interleukins for B lymphocytes. Survey of immunologic research 2: 210–212. Link: https://goo.gl/oFd6vC
- Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE (1999) The IL-4 receptor: signaling mechanisms and biologic functions. Annual review of immunology 17: 701–738. Link: https://goo.gl/S8qZyU
- Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, et al. (2012) An essential role for the Th2-type response in limiting tissue damage during helmit infection. National Institute of Health 18: 260–266. Link: https://goo.gl/H7VgAo
- Stockinger B, Veldhoen M (2007) Differentiation and function of Th17 T cells. Current Opinion in Immunology 19: 281–286. Link: https://goo.gl/AW7FZ9
- Adamopoulos IE, Sabokbar A, Wordsworth BP, Carr A, Ferguson DJ,et al. (2006) Synovial fluid macrophages are capable of osteoclast formation and resorption. J Pathol 208: 35-43. Link: https://goo.gl/y4fsre
- Cruz A, Khader SA, Torrado E, Fraga A, Pearl JE, et al. (2006) Cutting edge: IFN-gamma regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. Journal of immunology 177: 1416–1420. Link: https://goo.gl/B4Z5ex
- Dardalhon V, Korn T, Kuchroo VK, Anderson AC (2008) Role of Th1 and Th17 cells in organ-specific autoimmunity. Journal of Autoimmunity 31: 252–256. Link: https://goo.gl/wUuGWI
- Maddur MS, Miossec, P, Kaveri SV, Bayry, J (2012) Th17 cells: Biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. American Journal of Pathology 181: 8–18. Link: https://goo.gl/qpxQt7
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, et al. (2007) Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nature immunology 8: 639–646. Link: https://goo.gl/N9Wt8X
- Lexberg MH, Taubner A, Albrecht I, Lepenies I, Richter A, et al. (2010) IFN-γ and IL-12 synergize to convert in vivo generated Th17 into Th1/Th17 cells. European Journal of Immunology 40: 3017–3027. Link: https://goo.gl/DbMCDa
- Shen H, Goodall JC, Hill Gaston JS (2009) Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis and Rheumatism 60: 1647–1656. Link: https://goo.gl/W7zDH3b
- Lubberts E, Koenders MI, van den Berg WB (2005) The role of T-cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis research & therapy 7: 29–37. Link: https://goo.gl/LovCo2
- Wing K, Sakaguchi S (2010) Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nature immunology 11: 7–13. Link: https://goo.gl/bUc78x
- Dardalhon V, Korn T, Kuchroo, VK, Anderson AC (2008) Role of Th1 and Th17 cells in organ-specific autoimmunity. Journal of Autoimmunity 31: 252–256. Link: https://goo.gl/eo2p3Z
- Apostolou I, Von Boehmer H (2004) In vivo instruction of suppressor commitment in naive T cells. J Exp Med 199: 1401–1408. Link: https://goo.gl/wRc7Cc
- Curotto de Lafaille MA, Lafaille JJ (2009) Natural and Adaptive Foxp3+ Regulatory T Cells: More of the Same or a Division of Labor? Immunity 30: 626–635. Link: https://goo.gl/XrQz5h
- Yuan FL, Li X, Lu WG, Xu RS, Zhao YQ, et al. (2010) Regulatory T cells as a potent target for controlling bone loss. Biochemical and Biophysical Research Communications 402: 173–176. Link: https://goo.gl/6I8tgQ
- Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M (2006) Imbalance of regulatory T cells in human autoimmune diseases. Immunology 117: 289–300. Link: https://goo.gl/yQioPD
- Jones TG, Finkelman FD, Austen KF, Gurish MF (2010) T regulatory cells control antigen-induced recruitment of mast cell progenitors to the lungs of C57BL/6 mice. J Immunol 185: 1804–1811. Link: https://goo.gl/vah1qm
- Brahmachari S, Pahan K (2009) Suppression of regulatory T cells by IL-12p40 homodimer via nitric oxide. Journal of immunology 183: 2045–2058. Link: https://goo.gl/ZCIxBB
- Broere F, Apasov SG, Sitkovsky MV, Eden W Van (2011) A2 T cell subsets and T cell-mediated immunity. Principles of Immunopharmacology 15–27. Link: https://goo.gl/5H2Ubr
- Fehervari Z, Sakaguchi S (2006) Peacekeepers of the immune system. Sci Am 295: 56–63. Link: https://goo.gl/COv0zt
- Shevach EM, Stephens GL (2006) The GITR-GITRL interaction: co-stimulation or contrasuppression of regulatory activity? Nature Reviews. Immunology 6: 613–618. Link: https://goo.gl/jcUCeT
- Nataly Manjarrez Orduño, Christine Grimaldi, Betty Diamond B cell (2013) Kelley’s textbook of Rheumatology, 9th edition, Chapter 14 191-214. Link: https://goo.gl/xhqG9t
- Scott McComb, Aude Thiriot, Felicity C Stark, Lakshmi Krishnan (2013) Introduction to the Immune System. Methods Mol Biol. 1061:1-20. Link: https://goo.gl/YWh726
- Elhelu MA (1983) The role of macrophages in immunology. Journal of the National Medical Association 75: 314–317. Link: https://goo.gl/OeK0DP
- Ponath PD, Qin S, Post TW, Wang J, Wu L, et al. (1996) Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med 183: 2437-2448. Link: https://goo.gl/qHyAyQ
- Yang D, Qian Chen, Shao Bo Su, Ping Zhang, Kahori Kurosaka, et al. (2008) Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. The Journal of experimental medicine 205: 79–90. Link: https://goo.gl/y6iBpn
- Lord BI, Bronchud MH, Owens S, Chang J, Howell A, et al. (1989) The kinetics of human granulopoiesis following treatment with granulocyte colony-stimulating factor in vivo. Proceedings of the National Academy of Sciences 86: 9499–9503. Link: https://goo.gl/3UH6uf
- Owen CA, Campbell EJ (1999) The cell biology of leukocyte-mediated proteolysis. J Leukoc Biol 65: 137-150. Link: https://goo.gl/5ME9cF
- Murray J, Barbara JA, Dunkley SA, Lopez AF, Van Ostade X, et al. (1997) Regulation of neutrophil apoptosis by tumor necrosis factor-alpha: requirement for TNFR55 and TNFR75 for induction of apoptosis in vitro. Blood 90: 2772-2783. Link: https://goo.gl/pL18UN
- Tortorella C, Salvatore Pece, Emilio Jirillo, Salvatore Antonaci, Giuseppina Piazzolla, et al. (1998) Spontaneous and fas-induced apoptotic cell death in aged neutrophils. Journal of Clinical Immunology 18: 321–329. Link: https://goo.gl/xpJo45
- Galli SJ, Maurer M, Lantz CS (1999) Mast cells as sentinels of innate immunity. Current Opinion in Immunology 11: 53–59. Link: https://goo.gl/1ai9rt
- Galli SJ, Zsebo KM, Geissler EN (1994) The kit ligand, stem cell factor. Advances in immunology 55: 1–96. Link: https://goo.gl/7McAiQ
- Juurikivi A, Sandler C, Lindstedt KA, Mäki T, Eklund KK, et al (2005) Inhibition of c-kit tyro- sine kinase by imatinib mesylate induces apoptosis in mast cells in rheumatoid synovia: a potential approach to the treatment of arthritis. Ann Rheum Dis 64: 1126–1131. Link: https://goo.gl/DfVaLv s
- McClain C, Hill D, Schmidt J, Diehl AM (1993) Cytokines and alcoholic liver disease. Semin Liver Dis 13, 170–182. Link:
- Raphael I,Nalawade S, Eagar TN, Forsthuber TG (2015) T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 74: 5-17. Link: https://goo.gl/8OiFbf
- Mandal A, Viswanathan C (2015) Natural killer cells: In health and disease. Hematology/oncology and stem cell therapy 8: 47–55. Link: https://goo.gl/dL58Wc
- Pahan K (2011) Immunomodulation of experimental allergic encephalomyelitis by cinnamon metabolite sodium benzoate. Immunopharmacology and immunotoxicology 33: 586–593. Link: https://goo.gl/nnFI4Y
- Joseph M, Cohen (2014) The Health Benefits of Sodium Benzoate. (A Food Preservative). Link: https://goo.gl/hhgAOE
- Warrington RJ, Sauder PJ, McPhillips S (1986) Cell-mediated immune responses to artificial food additives in chronic urticaria. Clinical Allergy 16: 527–533. Link: https://goo.gl/zqbx0k
- Yadav A, Kumar A, Das M, Tripathi A (2016) Sodium benzoate, a food preservative, affects the functional and activation status of splenocytes at non cytotoxic dose. Food and Chemical Toxicology 88: 40–47. Link: https://goo.gl/dnVaWt
- Madhuchhanda Kundu, Susanta Mondal, Avik Roy, Jeffrey L. Martinson, Kalipada Pahan (2016) Sodium Benzoate, a Food Additive and a Metabolite of Cinnamon, Enriches Regulatory T Cells via STAT6-Mediated Upregulation of TGF- β. J Immunol 197: 3099-3110. Link: https://goo.gl/qk5Ipf
- Maier E, Kurz K, Jenny M, Schennach H, Ueberall F, et al. (2010) Food preservatives sodium benzoate and propionic acid and colorant curcumin suppress Th1-type immune response in vitro. Food and Chemical Toxicology 48: 1950–1956. Link: https://goo.gl/eLPp3N
- Brahmachari S, Jana A, Pahan K (2009) Sodium benzoate, a metabolite of cinnamon and a food additive, reduces microglial and astroglial inflammatory responses. Journal of immunology 183: 5917–5927. Link: https://goo.gl/UMhrFW
- Ciardi C, Jenny M, Tschoner A, Ueberall F, Patsch J, et al. (2012) Food additives such as sodium sulphite, sodium benzoate and curcumin inhibit leptin release in lipopolysaccharide-treated murine adipocytes in vitro. The British journal of nutrition 107: 826–833. Link: https://goo.gl/qccoMV
- Kruzel ML, Harari Y, Mailman D, Actor JK, Zimecki, M (2002) Differential effects of prophylactic, concurrent and therapeutic lactoferrin treatment on LPS-induced inflammatory responses in mice. Clinical and Experimental Immunology 130: 25–31. Link: https://goo.gl/hMZQtX
- Das L, Vinayak M (2014) Long-term effect of curcumin down-regulates expression of tumor necrosis factor-α and interleukin-6 via modulation of E26 transformation-specific protein and nuclear factor-κB transcription factors in livers of lymphoma bearing mice. Leukemia & lymphoma 55: 2627–2636. Link: https://goo.gl/gD4uKr
- Abuharfeil N, Sarsour E, Hassuneh M (2001) The effect of sodium nitrite on some parameters of the immune system. Food and Chemical Toxicology 39: 119–124. Link: https://goo.gl/KfnVNU
- Alyoussef A (2015) Clinical Research and Development Oral Sodium Nitrite Enhanced Oxidative Stress and Inflammatory Cytokines Levels in Testicular Tissue. 2: 1–5.
- Brahmachari S, Pahan K (2010) Myelin basic protein priming reduces the expression of Foxp3 in T cells via nitric oxide. J Immunol 184: 1799–1809. Link: https://goo.gl/QAY6Yx
- Bogdan C (2001) Nitric oxide and the immune response. Nat Immunol 2: 907–916. Link: https://goo.gl/wPvGhh
- Moutinho ILD, Bertges LC, Assis RVC (2007) Prolonged use of the food dye tartrazine (FD&C yellow no 5) and its effects on the gastric mucosa of Wistar rats. Braz J Biol 67: 141–145. Link: https://goo.gl/AbbTDD
- McCann D, Barrett A, Cooper A, Crumpler D, Dalen L, et al. (2007) Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebocontrolled trial. Lancet 370: 1560–1567. Link: https://goo.gl/8gHuHp
- Ashaolu JO, Ukwenya VO, Okonoboh A, Ghazal OK, Jimoh AAG (2011) DepartmentEffect of monosodium glutamate on hematological parameters in Wistar rats. International Journal of Medicine and Medical Sciences 3: 219–222. Link: https://goo.gl/wrXcpZ
- Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, et al. (2015) Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 519: 92-96. Link: https://goo.gl/izf8jm
- Yousf H, Tomar, GB, Srivastava RK Probiotics. Bone Health: It takes GUTS to Improve Bone Density. International Journal of Immunotherapy and Cancer Research 1: 5. Link: https://goo.gl/PJO6Pu
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
