International Journal of Immunotherapy and Cancer Research
Dalton’s Lymphoma as a Murine Model for Understanding the Progression and Development of T-Cell Lymphoma and Its Role in Drug Discovery
Raj Kumar Koiri1*, Aditi Mehrotra2 and Surendra Kumar Trigun2
2Biochemistry & Molecular Biology Lab, Department of Zoology, Banaras Hindu University, Varanasi - 221005, Uttar Pradesh, India
Cite this as
Koiri RK, Mehrotra A, Trigun SK (2017) Dalton’s Lymphoma as a Murine Model for Understanding the Progression and Development of T-Cell Lymphoma and Its Role in Drug Discovery. Int J Immunother Cancer Res 3(1): 001-006. DOI: 10.17352/2455-8591.000011Mouse models are irreplaceable tools for the study of carcinogenesis and the availability of rodent models have enabled rational screening of drugs. Hematological malignancies have been extensively studied in mouse models and broad range of lymphoid neoplasms has been reported in laboratory mice, occurring either spontaneously or after induction with radiation, chemicals or infection of newborn mice with leukemogenic viruses. Lymphomas are tumors that generally respond well to traditional therapies such as chemotherapy and radiotherapy. Dalton’s lymphoma is a transplantable T-cell lymphoma of spontaneous origin in thymus of murine host and has emerged as an interesting model for cancer research, because of its usefulness in pre-clinical system for evaluating new or known drugs in the treatment of various cancers and in drug discovery development process.
Introduction
The identification of novel clinically active agents has been central to progress in cancer chemotherapy. Animal models in general, and especially mouse models, are irreplaceable tools for the study of carcinogenesis and the availability of rodent models have enabled rational screening of drugs. The mouse shares anatomical, immunological and genomic similarities with humans and is the most accessible model system [1]. A goal in the studies of mouse lymphomas is to identify the human counterpart to the studied disease in order to understand the pathogenesis and evaluate new treatments for the disorder in humans. Hematological malignancies have been extensively studied in mouse models and broad range of lymphoid neoplasms has been reported in laboratory mice, occurring either spontaneously or after induction with radiation, chemicals or infection of newborn mice with leukemogenic viruses [2]. Lymphomas are tumors that generally respond well to traditional therapies such as chemotherapy and radiotherapy. However, for the patients that do not respond, or when tumors recur, new therapeutic approaches are warranted. Pre-clinical studies are important to screen the new compounds for potential effect against the disease. Murine lymphoma models are relatively inexpensive and easy to maintain; thus proven to be a useful tool in studies of lymphomagenesis and lymphoma treatment. Preclinical activity of an anti-tumor agent in a relevant in vivo system is a sine qua non for clinical testing. Multiple studies have been undertaken to assess the ability of preclinical animal activity to predict antitumor response in man.
Lymphoma: Hodgkin’s lymphoma and non-Hodgkin’s lymphoma
Lymphomas are a large and heterogeneous group of malignant diseases of lymphoid tissue, consisting of 70 different subtypes recognized within the new World Health Organisation (WHO) IV lymphoma classification system [3]. White blood cells (leukocytes), which constitute the cells of the immune system, are divided into lymphoid cells (T and B lymphocytes) and myeloid cells (granulocytes, monocytes and macrophages). Lymphoma is the broadest category of a family of related blood cancers, involving a group of cells, called the lymphocytes, which in turn make up the lymphatic system—a part of the immune system. It is a type of blood cancer that occurs when lymphocytes, which are white blood cells that help to protect the body from infection and diseases starts behaving abnormally, causing them to divide faster and may live longer than they are supposed to. Lymphoma may develop in many parts of the body, including the lymph nodes, spleen, bone marrow, blood and sometimes, non-hemopoetic tissues are also involved. Malignant lymphoma is the generic term given to tumors of the lymphoid system. These tumors are divided into two major categories: Hodgkin’s lymphoma and non-Hodgkin’s lymphoma (NHL); named after Thomas Hodgkin, who first described Hodgkin’s lymphoma in 1832 [4, 5]. Hodgkin’s lymphoma is characterized by the presence of an abnormal cell type, Reed-Sternberg cell (a B lymphocyte) which expresses B cell markers, such as CD20, but is absent in the second type of lymphomas, therefore referred to as non-Hodgkin’s lymphomas (NHL) [6,7]. Non-Hodgkin’s lymphoma is a heterogeneous group of malignancies characterized by an abnormal clonal proliferation of T cells, B cells, or both [8].
Both types can start in the lymphoid tissue (also called lymphatic tissue) and can spread to other organs. Lymphoid tissue is found in many places throughout the body, including lymph nodes, the thymus (found behind the chest bone and in front of the heart), the spleen (on the left side of the abdomen next to the stomach), the tonsils and adenoids, in the bone marrow, and scattered within other systems such as the digestive and respiratory systems. Patients with lymphoma when physically examined presents the symptoms of lymphadenopathy, splenopathy, enlarged liver and kidneys, pleural exudate, oedema, abdominal swelling, somnolence, tachypnea and tachycardia [9]. Lymphadenopathy can cause compression of other tissues like the ureter or spinal cord. Rapid tumour growth in aggressive lymphomas causes severe illness. Lymphomas can transform from non-aggressive lymphomas to aggressive lymphomas and therefore high-grade malignancies. Radiotherapy for treatment is not directly useful, and therefore patients receive chemotherapy, primarily Rituximab to CHOP (a combination of cyclophosphamide, vincristine, doxorubicin and prednisolone), followed by radiotherapy [8]. Each lymphoid neoplasm’s have a characteristic morphology and if well prepared adequately sized sections are available than it is possible to diagnose the type of lymphoma. However, due to many pitfalls in histological diagnosis of malignant lymphoma, cell surface phenotyping of lymphocytes is usually performed for classifying and diagnosing the type of lymphoma, usually performed on peripheral blood using flow cytometry or on tissues using immunoblotting, immunohistochemistry or ELISA. Flow cytometry is generally used to identify the markers on the surface of cells and provides the percentage of lymphoytes positive for a particular antigen and density of antigens; normal peripheral blood lymphocytes usually consist of ~ 10% B-cells, 80% T-cells and 10% NK-cells. Flow cytometry has proved very useful in making a specific diagnosis. Some of the commonly used markers and especially CD markers (cluster of differentiation/cluster designation) for B-cell, T-cell, monocytes and other cell types have been presented in Table 1.
Incidence of Non-Hodgkin’s lymphoma
In India among the males aged between 15–29 years, the three most common cancers are leukemia, lymphoma, and central nervous system tumors. Non-Hodgkin’s lymphoma is the 11th most common cancer in terms of incidence [10]. It is most frequent in high income countries, with rates more than twice those of middle- to low-income countries. It is usually fatal, with a 5 year survival rate of less than 35 percent. It is not a single cancer, but rather a wide group of cancers (including entities such as Burkitt’s lymphoma and diffuse large B-cell lymphoma), each with a distinct geographical distribution, development path, age profile and prognosis. The incidence rates of Non-Hodgkin’s lymphoma have risen dramatically in the last 30 years, particularly in developed countries, including Western Europe, North America and Australia [11]. Non Hodgkins Lymphoma, which was once considered rare, has slowly grown to the fifth most common cancer (incidence of 19.1 per 100,000 in USA) in the world [12]. About 65,500 cases of non-Hodgkins lymphoma were expected to be diagnosed in the United States in 2010 [13]. Non-Hodgkins lymphoma occurs in individuals at virtually all ages, but it is uncommon in children. The incidence of NHL increases with age [13]. In the 20- to 24-year age-group, 2.4 cases occur per 100,000 persons. The rate increases almost 20-fold to 46.3 cases per 100,000 individuals by age 60 to 64 years, and over 40- fold to more than 100 cases per 100,000 persons after age 75. The age adjusted incidence of NHL has increased more than 82 percent from 1975 to 2007, an average annual increase of about 2.7 percent. The reasons for this increase are not certain, and there are probably multiple causes.
In India its incidence is on the upsurge with the current figure standing at 5.1 per 100,000 in urban registries. The incidence of lymphomas is still growing, and in the future this group of malignancies will form a quantitatively remarkable subtype of malignant diseases. In many lymphoma subtypes, the prognosis is good and the treatment results are continuously improving due to new treatment modalities. However, some lymphoma patients still succumb to their disease and certain most aggressive subtypes of lymphoma are often beyond curative treatment [14-17]. NHL being a systemic disease, a systemic approach like chemotherapy has been considered to be more appropriate and is treated with chemotherapy, and in some cases radiotherapy and/or bone marrow transplantation, and can be curable depending on the histology, type, and stage of the disease [18].
T cell lymphomas
Lymphoma is a malignancy of the immune system. In normal T-cell development, T-cell progenitors are generated in the bone marrow and then migrate to the thymus gland. The T cells mature in the thymic cortex and T cells recognizing self-antigens are eliminated in a process called negative selection. The different developmental stages can be recognized by the expression of certain cell surface molecules. Cortical thymocytes are initially double negative for the cell surface molecules CD4 and CD8. During the maturation process, the T-cell goes from the CD4/CD8 double negative stage to a double positive stage and finally expresses only CD4 or CD8. In the CD4 or CD8 single positive stage, the T-cell is considered mature and it migrates to the peripheral/secondary lymphoid tissue [19-21]. The bone marrow and the thymus gland are considered primary lymphoid organs whereas spleen, lymph nodes and mucosa associated lymphoid tissue are the secondary lymphoid organs where immune responses are initiated. T cells residing in the medullary thymus have the phenotype of mature T cells. CD4 positive T cells are called helper T cells and are subdivided into Th1 and Th2 cells based on their pattern of cytokine production. They provide help to other cells in the immune system; Th1 aid other T cells and macrophages whereas Th2 cells help the B cells in antibody production. CD8 positive T cells act as cytotoxic T cells with the ability to directly kill infected or otherwise targeted cells [19-21].
T-cell lymphomas are clonal tumors of immature or mature T lymphocytes at various stages of differentiation. They account for only 10-12% of all NHLs, the rest being of B-cell origin. The clinical presentation of lymphomas is often a swollen lymph node in the neck, axilla or groin, but several other presentations are common such as abdominal or mediastinal masses or extranodal manifestations. T-cell lymphomas are generally associated with inferior outcome than lymphomas of B-cell origin [22] and continuous effort is put into finding new treatment modalities or efficient combinations of existing treatments [23,24]. According to World Health Organization (WHO, 2008) classification of tumors of hematopoietic and lymphoid tissue; T-cell lymphomas are divided into T-acute lymphoblastic leukemia/ lymphoma (T-ALL/LBL) originating from immature T cells in bone marrow or thymus, and mature T-cell lymphomas arising in peripheral lymphoid organs.
Daltons Lymphoma
Daltons lymphoma are widely used as interesting model for cancer research, because of its usefulness in pre-clinical system for evaluating new or known drugs in the treatment of various cancers. Dalton’s lymphoma is a transplantable T-cell lymphoma of spontaneous origin in thymus of murine host [25, 26]. During the late tumour bearing stages, DL growth has been shown to be associated with a concomitant inhibition of humoral and cell mediated immune responses involving the abrogated functions of macrophages, B and T cells [27, 28] and with an involution of thymus with a massive depletion of immature CD4+CD8+ and mature CD4+CD8- and CD4-CD8+ thymocytes by the process of apoptotic cell death resulting in an alteration in the distribution of T-cell sub populations in the thymus [29]. Immunophenotypic characterization of the lymphoma has revealed it to be of T-cell origin with expression of CD4 and/or CD8 and T-cell receptor (TCR) αβ. T-cells obtained from DL bearing mice have been found to be sensitive for DL derived factors like DL ascitic fluid, DL conditioned medium or tumor serum and defective in their ability to produce IFN-γ and IL-2. DL cells have been reported to produce enhanced amounts of IL-10, which is a well-established TH2 specific immuno suppressive cytokine, thus indicating an alteration in the TH specific cytokine profile with the progression of DL growth suggesting immune deviation toward a TH2 type response. Predominance of the TH2 type cytokine pattern indicates an inhibition of cellular immune response [29]. Thus, ascitic growth of a transplantable T-cell lymphoma of spontaneous origin leads to an impairment of T-cells by diminishing their proliferative abilities and effector functions through an altered profile of immunoregulatory cytokines like IFN-γ, IL-10 & IL-2. However, molecular basis of T-cell dysfunction still remains to be elucidated.
In cancer drug development, the animal model is selected to demonstrate the cytotoxic effect of the drug or biological agent on the tumor passage in that model system. In selecting the best model system, consideration is given to the genetic stability and heterogeneity of the transplanted cell line, its immunogenecity within the host animal, and the appropriate biologic endpoint (local growth, metastasis, survival). In this respect, Dalton’s lymphoma is a very good model system, active components of a large number of natural plant products have been studied with reproducible biologic endpoint like local growth and predictable survival period [30-33]. Dalton’s lymphoma is an established transplantable tumor model which is well characterized and reproducible, and traditionally have been the foundation of drug development.
Due to effective biodistribution and multimodal cellular actions, during recent past, ruthenium and certain other metal complexes like platinum, copper and gold have drawn much attention as next generation anticancer agents [34,35]. In this respect Dalton’s lymphoma has been successfully used to evaluate the anticancer effect and subsequently the mechanism of action of newly synthesized metal complexes of ruthenium [36-38], platinum [39], copper [40] and gold complexes. Recently Sriram et al., 2010, has demonstrated the antitumor activity of silver nanoparticles using Dalton’s lymphoma ascites as a tumor model [32].
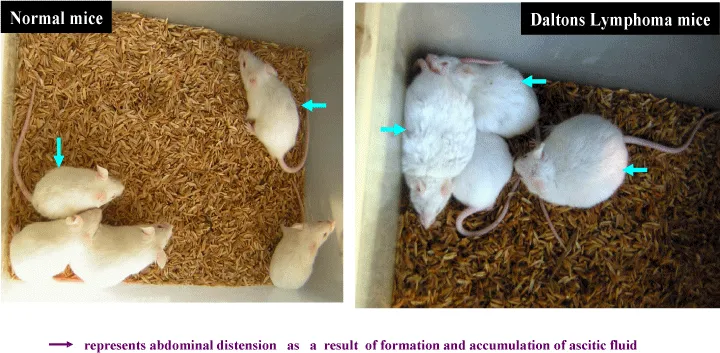
Dalton’s lymphoma (DL) ascites tumorigenesis model in mice provides a convenient model system to study such effects within a short time [29]. Following transplantation of DL ascites cells into the abdominal cavity of healthy recipient mice, tumorigenesis begins immediately and aggressively [26]. The recipient or transformed mice usually survive up to ~3 weeks [36,41]. Dalton’s lymphoma can be easily maintained in laboratory in ascites by serial transplantation in mice by intraperitoneal injection of 5x105 cells/mouse [36]. A range of parameters can be used to evaluate drug effect on tumors in this model. Tumor volume and changes in body weight are simple and easily reproducible parameters (Figure 1). Morphologic changes and alterations in tumor immunogenecity or invasiveness are other markers of response. In addition many specific assays have been developed for the measurement of treatment effects on tumors. Another parameter that can be used to assess the effect of a drug on tumor in the animal model is the survival time. Survival time is an obvious endpoint, since it combines the sum total of interactions between tumor, drug and host. Since drug toxicity and tumor growth both have independent effects on survival, a judgement can be made about therapeutic index.
Anti-cancer property of a number of drugs and their subsequent mechanism of action and the pathways implicated has been elucidated using this murine model. Alcoholic extract of Curculigo orchioides has been reported to effectively down regulate Dalton’s lymphoma ascites induced solid tumour and Ehrlich ascites carcinoma induced ascites tumour development and enhance the tumoricidal activity of mice peritoneal macrophages [42]. Antitumor activity of ethanolic extract of Cnidoscolus chayamansa [43], active fraction of Emilia sonchifolia [44], Crocin from Kashmiri saffron (Crocus sativus) [45], Aegle marmelos [46], Ganoderma lucidum [47], Chondrococcus hornemanni and Chondrococcus hornemanni [48], steroid positive compound from Zornia diphylla [49], Leucas aspera [50], phthalmustine [51], atorvastatin, an inhibitor of HMG-CoA reductase [52] have been demonstrated against Dalton’s ascitic lymphoma in mice. Abrin isolated from seeds of Abrus precatorius [53], extract of Abrus precatorius [54] and Drosera indica [55] have been reported to inhibit cell growth and induce apoptosis in Dalton’s lymphoma ascites cells through activation of caspase-3 and downregulation of Bcl-2. Antiproliferative activity of benzophenone tagged pyridine analogues towards activation of caspase activated DNase mediated nuclear fragmentation has been studied in Dalton’s lymphoma [56]. Activation of p53 mediated glycolytic inhibition-oxidative stress-apoptosis pathway in Daltons lymphoma has been reported by a ruthenium (II)-complex containing 4-carboxy N-ethylbenzamide [57]. Thus, Dalton’s lymphoma has emerged as an important murine model for understanding the progression and development of T-cell lymphoma and is playing an important role in drug discovery and development.
This work was financially supported by a project from UGC-Faculty Research Promotion Scheme (FRPS) & SERB, Govt. of India, sanctioned to RKK. AM and SKT thanks Department of Zoology, Banaras Hindu University and RKK acknowledges, Department of Zoology, Dr. Harisingh Gour Central University for providing infrastructural facilities and financial support.
- Bernardi R, Grisendi S, Pandolfi PP (2002) Modelling haematopoietic malignancies in the mouse and therapeutical implications. Oncogene 21: 3445-3458. Link: https://goo.gl/NhfpGW
- Pattengale PK, Taylor CR (1983) Experimental models of lympho proliferative disease. The mouse as a model for human non-Hodgkin's lymphomas and related leukemias. Am J Pathol 113: 237-265. Link: https://goo.gl/p0Gk3m
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, et al. (2008) World Health Organization Classification of tumours: pathology and genetics, tumours of haematopoietic and lymphoid tissue. IARC Press. 243-244. Link: https://goo.gl/T5HgbA
- Hodgkin T (1832) On some morbid appearances of the absorbent glands and spleen. Med Chir Trans 17: 68-114. Link: https://goo.gl/BY5S7X
- Wilks S (1865) Cases of enlargement of the lymphatic glands and spleen (or Hodgkin's disease) with remarks. Guy's Hosp Rep 11: 56–67. Link: https://goo.gl/7xCF0H
- Sternberg C (1898) Über eine eigenartige unter dem Bilde der Pseudoleukämie verlaufende Tuberkolose des lymphatischen Apparates. Zeitschrift für Heilkunde 19: 21-90.
- Reed D (1902) On the pathological changes in Hodgkin’s disease with special reference to its relation in tuberculosis. John Hopkins Hospital Reports 10: 133-193. Link: https://goo.gl/gWuET4
- Evans LS, Hancock BW (2003) Non-Hodgkin lymphoma. Lancet 362: 139-146. Link: https://goo.gl/HFKtsR
- Chan FH, Carl D, Lyckholm LJ (2009) Severe lactic acidosis in a patient with B-cell lymphoma: a case report and review of the literature. Case Rep Med 534561. Link: https://goo.gl/WYKd30
- Yeole BB (2008) Trends in the Incidence of Non-Hodgkin’s Lymphoma in India. Asian Pac J Cancer Prev 9: 433-436. Link: https://goo.gl/hP80EU
- Parkin DM, Bray F, Ferlay J, Pisani P (2005). Global cancer statistics, 2002. CA Cancer J Clin 55: 74-108. Link: https://goo.gl/lgZmU8
- Cerny T, Gillessen S (2002) Advances in the treatment of non-Hodgkin’s lymphoma. Ann Oncol 13: 211-216. Link: https://goo.gl/iUnYjL
- Surveillance, Epidemiology, and End Results [SEER] Program, 2010. Link: https://goo.gl/J519W9
- McLaughlin P, Fuller L, Redman J, Hagemeister F, Durr E, et al. (1991) Stage I-II low-grade lymphomas: a prospective trial of combination chemotherapy and radiotherapy. Ann Oncol 2: 137-140. Link: https://goo.gl/Cr8Jv9
- Miller TP, Dahlberg S, Cassady JR, Adelstein DJ, Spier CM, et al. (1998) Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate and high-grade non-Hodgkin′s lymphoma. N Engl J Med 339: 21-26. Link: https://goo.gl/pRNQfo
- Gianni AM, Bregni M, Siena S, Brambilla C, Di Nicola M, et al. (1997) High-dose chemotherapy and autologous bone marrow transplantation compared with MACOP-B in aggressive B-cell lymphoma. N Engl J Med 336: 1290-1297. Link: https://goo.gl/9fZCrK
- Zinzani PL (2005) Lymphoma: diagnosis, staging, natural history, and treatment strategies. Semin Oncol 32: S4-10. Link: https://goo.gl/bGbaM3
- Parham P (2005) The immune system. New York: Garland Science. 414. Link: https://goo.gl/NpYeCA
- Fehling HJ, Krotkova A, Saint-Ruf C, von Boehmer H (1995) Crucial role of the pre-T-cell receptor α gene in development of αβ but not γδ T cells. Nature 375: 795-798. Link: https://goo.gl/RUImG2
- Capone M, Hockett RD and Zlotnik A (1998) Kinetics of T cell receptor β, γ, and δ rearrangements during adult thymic development: T cell receptor rearrangements are present in CD44+CD25+ Pro-T thymocytes. Proc Natl Acad Sci USA 95: 12522-12527. Link: https://goo.gl/k3V1VQ
- Prockop S, Petrie HT (2000) Cell migration and the anatomic control of thymocyte precursor differentiation. Semin Immunol 12: 435-444. Link: https://goo.gl/IuYw3A
- Gisselbrecht C, Gaulard P, Lepage E, Coiffier B, Brière J, et al. (1998) Prognostic significance of T-cell phenotype in aggressive non-Hodgkin's lymphomas. Blood 92: 76-82. Link: https://goo.gl/81dGTN
- Reimer P, Rüdiger T, Geissinger E, Weissinger F, Nerl C, et al. (2009) Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol 27: 106-113. Link: https://goo.gl/o827Yk
- Enblad G, Hagberg H, Erlanson M, Lundin J, MacDonald AP, et al. (2004) A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood 103: 2920-2924. Link: https://goo.gl/ZsLaiX
- Klein G (1951) Comparative studies of mouse tumors with respect to their capacity for growth as ‘ascitic tumor’ and their average nucleic acid content. Exp Cell Res 2: 518–524. Link: https://goo.gl/P794el
- Goldie H, Felix MD (1951) Growth characteristics of free tumor cells transformed serially in the peritoneal fluid of mouse. Cancer Res 11: 173–180. Link: https://goo.gl/xMMXld
- Kumar A, Singh SM, Parajuli P (1994) Modulatory effect of Dalton's lymphoma cells on the production of reactive nitrogen intermediates, interleukin-1 and tumor necrosis factor by murine peritoneal macrophages. Neoplasma 41: 363-369. Link: https://goo.gl/SQ3xaR
- Parajuli P, Singh SM, Kumar A, Sodhi A (1997) Alterations in the tumoricidal function of murine tumor-associated macrophages during progressive growth of a tumor in vivo. Cancer J 10: 222–228. Link: https://goo.gl/HPiyow
- Shanker A, Singh SM, Sodhi A (2000) Ascitic growth of a spontaneous transplantable T cell lymphoma induces thymic involution 1. Alterations in the CD4/CD8 distribution in thymocytes. Tumor Biol 21: 288-298. Link: https://goo.gl/u3Cy6F
- Ajith TA, Janardhanan KK (2003) Cytotoxic and antitumor activities of a polypore macrofungus, Phellinus rimosus (Berk) Pilat. J Ethnopharmacol 84: 157–162. Link: https://goo.gl/By2wnD
- Christina AJM, Joseph GD, Packialakshmi M, Kothai R, Robert SJH, et al. (2004) Anticarcinogenic activity of Withania somnifera Dunal against Dalton’s ascitic lymphoma. J Ethnopharmacol 93: 359–361. Link: https://goo.gl/1DHSJJ
- Sriram MI, Kanth SBM, Kalishwaralal K, Gurunathan S (2010) Antitumor activity of silver nanoparticles in Dalton's lymphoma ascites tumor model. Int J Nanomedicine 5: 753–759. Link: https://goo.gl/lV9XbB
- Jagethia GC, Venkatesh P, Baliga MS (2005) Aegle marmelos (L.) Correa inhibits the proliferation of transplanted Erlich Ascites Carcinoma in mice. Biol Pharm Bull 28: 58-64. Link: https://goo.gl/yOfCjY
- Trigun SK, Koiri RK, Mishra L, Dubey SK, Singh S, et al. (2007) Ruthenium complex as enzyme modulator: modulation of lactate dehydrogenase by a novel ruthenium(II) complex ontaining 4-carboxy N-ethylbenzamide as a ligand. Current Enzyme Inhibit 3: 243-253. Link: https://goo.gl/TWKuqZ
- Koiri RK, Mehrotra A, Trigun SK (2013) Targetting cancer with Ru(III/II)-phosphodiesterase inhibitor adducts: a novel approach in the treatment of cancer. Med Hypotheses 80: 841-846. Link: https://goo.gl/pacGi2
- Koiri RK, Trigun SK, Mishra L, Pandey K, Dixit D, et al. (2009) Regression of Dalton's lymphoma in vivo via decline in lactate dehydrogenase and induction of apoptosis by a ruthenium(II)-complex containing 4-carboxy N-ethylbenzamide as ligand. Invest New Drugs 27: 503-516. Link: https://goo.gl/9SgKQF
- Koiri RK, Trigun SK (2011) Dimethyl sulfoxide activates tumor necrosis factorα-p53 mediated apoptosis and down regulates d-fructose-6-phosphate-2-kinase and lactate dehydrogenase-5 in Dalton’s lymphoma in vivo. Leuk Res 35: 950–956. Link: https://goo.gl/YhdthP
- Prajapati R, Dubey SK, Gaur R, Koiri RK, Maurya BK, et al. (2010) Structural characterization and cytotoxicity studies of ruthenium(II)–dmso–chloro complexes of chalcone and flavone derivatives. Polyhedron 29: 1055–1061. Link: https://goo.gl/unczk8
- Kumari N, Maurya BK, Koiri RK, Trigun SK, Saripella S, et al. (2011) Cytotoxic activity, cell imaging and photocleavage of DNA induced by a Pt(II) cyclophane bearing 1,2 diamino ethane as a terminal ligand. MedChemCommun 2: 1208–1216. Link: https://goo.gl/vPBK2S
- Namrata Dixita, R K. Koirib, B.K Mauryab, S K Trigunb, Claudia Höbartner, (2011) One pot synthesis of Cu(II) 2,2'-bipyridyl complexes of 5-hydroxy-hydurilic acid and alloxanic acid: synthesis, crystal structure, chemical nuclease activity and cytotoxicity. J Inorg Biochem 105: 256-267. Link: https://goo.gl/hR5YTh
- Devi BJ, Sharan RN (2006) Progressive reduction in poly-ADP-ribosylation of histone proteins during Dalton’s lymphoma induced ascites tumorigenesis in mice. Cancer Lett 238: 135–141. Link: https://goo.gl/qnNzab
- Murali VP, Kuttan G (2014) Alcoholic extract of Curculigo orchioides Gaertn. Effectively down regulates DLA and EAC induced tumour development and enhance the tumoricidal activity of mice peritoneal macrophages. Amelia Res Bull 3: 75-82. Link: https://goo.gl/rgtsY5
- Pillai K, Narayanan N, Chidambaranathan N, Mohamed Halith, Jayaprakash S (2012) Antitumor activity of ethanolic extract of CNIDOSCOLUS CHAYAMANSA MCVAUGH against Dalton’s Ascitic Lymphoma in Mice, Int J Pharm Pharm Sci 4: 647-652. Link: https://goo.gl/BftMDT
- Shylesh BS, Nair SA, Subramoniam A (2005) Induction of cell-specific apoptosis and protection from Dalton's lymphoma challenge in mice by an active fraction from Emilia sonchifolia. Indian J Pharmacol 37: 232-237. Link: https://goo.gl/up6kZJ
- Bakshi HA, Sam S, Feroz A, Ravesh Z, Shah GA, et al. (2009) Crocin from Kashmiri saffron (Crocus sativus) induces in vitro and in vivo xenograft growth inhibition of Dalton's lymphoma (DLA) in mice. Asian Pac J Cancer Prev 10: 887-890. Link: https://goo.gl/MsivBx
- Chockalingam V, Kadali SS, Gnanasambantham P (2012) Antiproliferative and antioxidant activity of Aegle marmelos (Linn.) leaves in Dalton's Lymphoma Ascites transplanted mice. Ind J Pharma 44: 225-229. Link: https://goo.gl/uyvO3N
- Smina TP, Mathew J, Janardhanan KK (2016) Ganoderma lucidum total triterpenes attenuate DLA induced ascites and EAC induced solid tumours in Swiss albino mice. Cell Mol Biol 62: 55-59. Link: https://goo.gl/zwsPTP
- Subbiah M, Sundaresan B (2012) Antitumor activity of Chondrococcus hornemanni and Chondrococcus hornemanni on Dalton’s lymphoma ascites in mice. Bangladesh J Pharmacol 7: 173-177. Link: https://goo.gl/fCK0xB
- Arunkumar R, Nair SA, Subramoniam A (2012) Induction of cell-specific apoptosis and protection of mice from cancer challenge by a steroid positive compound from Zornia diphylla (L.) Pers. J Pharmacol Pharmacother 3: 233-241. Link: https://goo.gl/ooqOF0
- Augustine BB, Dash S, Lahkar M, Sarma U, Samudrala PK, et al. (2014) Leucas aspera inhibits the Dalton's ascitic lymphoma in Swiss albino mice: A preliminary study exploring possible mechanism of action. Pharmacogn Mag 10: 118-124. Link: https://goo.gl/36IALv
- Bhattacharya S, Sanyal U, Ganguly C, Das S (1994) Evaluation of phthalmustine, a new anticancer compound The effect on Dalton's ascitic lymphoma in mice. Neoplasma 41: 35-38. Link: https://goo.gl/yZdrCX
- Ajith TA, Anu V, Riji T (2008) Antitumor and apoptosis promoting properties of atorvastatin, an inhibitor of HMG-CoA reductase, against Dalton's Lymphoma Ascites tumor in mice. J Exp Ther Oncol 7: 291-298. Link: https://goo.gl/ufR70f
- Ramnath V, Rekha PS, Kuttan G, Kuttan R (2009) Regulation of Caspase-3 and Bcl-2 expression in Dalton's Lymphoma ascites cells by Abrin. Evid Based Complement Alternat Med 6: 233-238. Link: https://goo.gl/veegYJ
- Harikumar KB, Kuttan G, Kuttan R (2009) Abrus precatorius inhibits cell growth and induces apoptosis in Dalton's lymphoma ascites cells through activation of caspase-3 and downregulation of Bcl-2. Integr Cancer Ther 8: 190-194. https://goo.gl/3JoCZJ
- Asirvatham R, Christina AJM (2013) Anticancer activity of Drosera indica L., on Dalton’s Lymphoma Ascites (DLA) bearing mice. J Intercult Ethnopharmacol 2: 9-14. Link: https://goo.gl/w8gGcW
- Al-Ghorbani M, Thirusangu P, Gurupadaswamy HD, Girish V, Shamanth NHG, et al. (2016) Synthesis and antiproliferative activity of benzophenone tagged pyridine analogues towards activation of caspase activated DNase mediated nuclear fragmentation in Dalton's lymphoma. Bioorg Chem 65: 73-81. Link: https://goo.gl/5jhqzZ
- Koiri RK, Trigun SK, Mishra L (2015) Activation of p53 mediated glycolytic inhibition-oxidative stress-apoptosis pathway in Dalton's lymphoma by a ruthenium (II)-complex containing 4-carboxy N-ethylbenzamide. Biochimie 110: 52-61. Link: https://goo.gl/4Ya11b
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
